Page 183 - Battery Reference Book
P. 183
Reserve type 1013
In recent years many companies have attempted, 75
successfully, to replace the zinc can anode of the
carbon-zinc primary cell with magnesium to take r
I
advantage of magnesium's greater electrochemical ._ 50
$
potential. The magnesium cell has an open-circuit volt- z
age of around 2.0 V, compared to 1.5 V for the zinc dry
cell. In addition to this higher voltage, the magnesium
dry cell has several other advantages over the conven-
tional dry cell. It has the capability of giving twice the 55 "C
gravimetric energy density (W fig) of the dry cell at 71 "C
medium dischmge rates (10-50 h). I I I I I 1
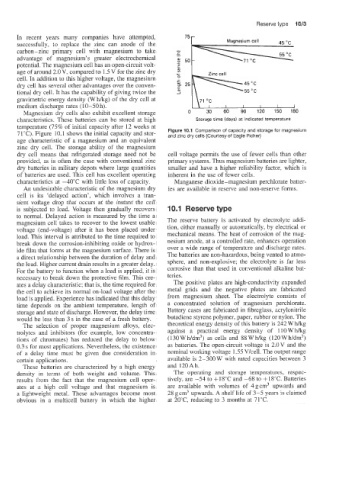
Magnesium dry cells also exhibit excellent storage 0 30 60 90 120 150 180
characteristics;. These batteries can be stored at high Storage time (days) at indicated temperature
temperature ('75% of initial capacity after 12 weeks at
71°C). Figure 10.1 shows the initial capacity and stor- Figure 10.1 Comparison of capacity and storage for magnesium
and zinc dry cells (Courtesy of Eagle Picher)
age characteristic of a magnesium and an equivalent
zinc dry cell. The storage ability of the magnesium
dry cell means that refrigerated storage need not be cell voltage permits the use of fewer cells than other
provided, as is often the case with conventional zinc primary systems. Thus magnesium batteries are lighter,
dry batteries in military depots where large quantities smaller and have a higher reliability factor: which is
of batteries are used. This cell has excellent operating inherent in the use of fewer cells.
characteristics at -40°C with little loss of capacity. Manganese dioxide-magnesium perchlorate batter-
An undesirable characteristic of the magnesium dry ies are available in reserve and non-reserve forms.
cell is its 'dlelayed action', which involves a tran-
sient voltage drop that occurs at the instant the cell
is subjected to load. Voltage then gradually recovers 10.1 Reserve type
to normal. Delayed action is measured by the time a
magnesium cell takes to recover to the lowest usable The reserve battery is activated by electrolyte addi-
voltage (end-voltage) after it has been placed under tion, either manually or automatically, by electrical or
load. This interval is attributed to the time required to mechanical means. The heat of corrosion of the mag-
break down tlne corrosion-inhibiting oxide or hydrox- nesium anode, at a controlled rate, enhances operation
ide film that forms at the magnesium surface. There is over a wide range of temperature and discharge rates.
a direct relationship between the duration of delay and The batteries are non-hazardous, being vented to atmo-
the load. Highler current drain results in a greater delay. sphere, and non-explosive; the electrolyte is far less
For the battery to function when a load is applied, it is corrosive than that used in conventional alkaline bat-
necessary to break down the protective film. This cre- teries.
ates a delay characteristic; that is, the time required for The positive plates are high-conductivity expanded
the cell to achieve its normal on-load voltage after the metal grids and the negative plates are fabricated
load is applied. Experience has indicated that this delay from magnesium sheet. The electrolyte consists of
time depends on the ambient temperature, length of a concentrated solution of magnesium perchlorate.
storage and state of discharge. However, the delay time Battery cases are fabricated in fibreglass, acrylonitrile
would be less than 3 s in the case of a fresh battery. butadiene styrene polymer, paper, rubber or nylon. The
The selection of proper magnesium alloys, elec- theoretical energy density of this battery is 242 W fig
trolytes and inhibitors (for example, low concentra- against a practical energy density of 110 W hkg
tions of chromates) has reduced the delay to below (130Wh/dm3) as cells and 88Whkg (120Wh/dm3)
0.3 s for most applications. Nevertheless, the existence as batteries. The open-circuit voltage is 2.0V and the
of a delay time must be given due consideration in nominal working voltage 1.55 V/cell. The output range
certain applications. available is 2-300 W with rated capacities between 3
These batteries are characterized by a high energy and 120Ah.
density in terns of both weight and volume. This The operating and storage temperatures, respec-
results from the fact that the magnesium cell oper- tively, are -54 to +18T and -68 to +18"@. Batteries
ates at a high cell voltage and that magnesium is are available with volumes of 4gcm3 upwards and
a lightweight metal. These advantages become most 28 g cm3 upwards. A shelf life of 3-5 years is claimed
obvious in a multicell battery in which the higher at 20T, reducing to 3 months at 71°C.