Page 219 - Battery Reference Book
P. 219
Chemical reactions during battery cycling 18/3
68.1 Chemical reactions during of plates, the extra one being a negative. Therefore, in
battery cycling a 13-plate cell. there will be seven negative and six
positive plates.
The basic cell reactions in the traditional lead-acid Contact between plates of opposite polarity must be
battery are as flollows: avoided, to prevent short-circuiting. This is normally
achieved by inserting the negative plate into a
discharge microporous envelope. Microporous envelopes have
PbOz + Pb + 2H2SO4 2FbSO4 + 2H20 (18.1) good insulating properties but low electrical resistance,
charge
thus allowing a free flow of ions and diffusion of
The reaction at the positive electrode: electrolyte.
The element is manufactured by assembling positive
discharge and negative plates alternately, zt the same time plac-
PbOz + 3H+ + NSO4 + 2e __i 2Hz0 + PbSOd(l8.2) ing the negative plate into the separator sleeve. The
charge
positive plates are interconnected by welding in posi-
At the negative electrode: tion a precast terminal post; the negative plates are
similarly connected. The cell is completed by he&-
discharge sealing the lid in position on the box.
Pb + HSO; W PbSO4 + H+ + 2E (18.3) Cells are said to be in series when the positive pole
charge
of one cell is connected to the negative pole of the
When the cell is recharged, the primary reaction tak- adjacent one, and this arrangement is continued for
ing place is as shown in Equation 18.1. Finely divided any desired number of cells. The voltage of cells in
particles of lead sulphate are being electrochemically series is additive. The capacity in ampere hours of the
converted to sponge lead at the negative electrode and battery will, however, still be that of a single cell. Cells
lead dioxide at the positive by the charging source driv- are connected in parallel when all the positive poles are
ing current through the battery. As the cell approaches joined together and all the negative poles are similarly
complete recharge, where the majority of the lead sul- connected. The voltage of cells so connected is that of
phate has been converted to lead and lead oxide, the a single cell, but the capacity of the combination is the
overcharge reactions begin. For typical lead-acid cells, sum of the individual cell capacities.
the result of these reactions is the production of hydro- Cell boxes were traditionally made from hard rub-
gen and oxygen gas and subsequent loss of water. ber, but there is now an increasing use of plastic
Further details of the influence of theory on the materials. Polypropylene is one such material. It has
design of lead-acid batteries is given below. This dis- the advantage of being more robust, and it allows a
cussion refers particularly to a motive power lead-acid polypropylene heat-sealed joint to be made between
battery. The same general principles apply to any con- cell box and lid, creating a significant improvement
ventional open type of lead-acid battery. over the box-to-lid seals previously used.
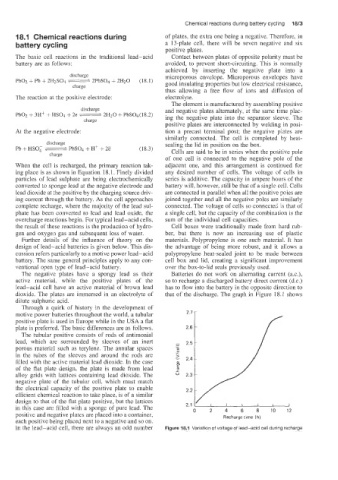
The negative plates have a spongy lead as their Batteries do not work on alternating current (a.c.),
active material, while the positive plates of the so to recharge a discharged battery direct current (d.c.)
lead-acid cell have an active material of brown lead has to flow into the battery in the opposite direction to
dioxide. The plates are immersed in an electrolyte of that of the discharge. The graph in Figure 18.1 shows
dilute sulphuric acid.
Through a quirk of history in the development of
motive power batteries throughout the world, a tubular 2.7 [
positive plate is used in Europe while in the USA a flat
plate is preferred. The basic differences are as follows. 2.6
The tubular positive consists of rods of antimonial
lead, which are surrounded by sleeves of an inert I 2.5
-
porous material such as terylene. The annular spaces -
..
W
in the tubes of the sleeves and around the rods are >
1 2.4
filled with the active material lead dioxide. In the case
P
of the flat plate design, the plate is made from lead 1
alloy grids with lattices containing lead dioxide. The 2.3
negative plate of the tubular cell, which must match
the electrical capacity of the positive plate to enable 2.2
efficient chemical reaction to talte place, is of a similar
design to that of the flat plate positive, but the lattices
I
I
I
in this case are filled with a sponge of pure lead. The 2.1 0 2 4 I 6 8 I 10 12
positive and negative plates are placed into a container, Recharge time (h)
each positive being placed next to a negative and so on.
In the lead-acid cell, there are always an odd number Figure 18.1 Variation of voltage of lead-acid cell during recharge