Page 222 - Battery Reference Book
P. 222
18/6 Lead-acid secondary batteries
lead-acid battery loses about half its initial capacity as power tools, portable radios, lamps, electronic,
during 3 months at 2WC, the same decrease would take communications and medical equipment, alarms and
about 16 months in the case of a 0.08% calcium-lead emergency lighting equipment.
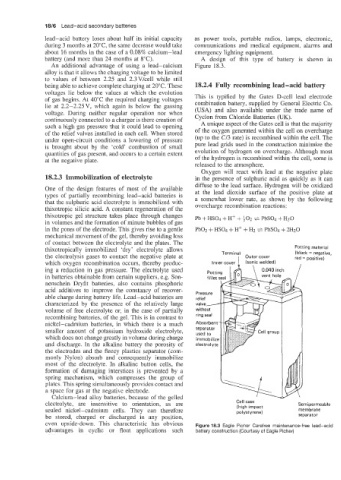
battery (and more than 24 months at 8°C). A design of this type of battery is shown in
An additional advantage of using a lead-calcium Figure 18.3.
alloy is that it allows the charging voltage to be limited
to values of between 2.25 and 2.3V/cell while still
being able to achieve complete charging at 20°C. These 18.2.4 Fully recombining lead-acid battery
voltages lie below the values at which the evolution This is typified by the Gates D-cell lead electrode
of gas begins. At 40°C the required charging voltages combination battery, supplied by General Electric Co.
lie at 2.2-2.25V, which again is below the gassing
(USA) and also available under the trade name of
voltage. During neither regular operation nor when Cyclon from Chloride Batteries (UK).
continuously connected to a charger is there creation of
such a high gas pressure that it could lead to opening A unique aspect of the Gates cell is that the majority
of the relief valves installed in each cell. When stored of the oxygen generated within the cell on overcharge
under open-circuit conditions a lowering of pressure (up to the C/3 rate) is recombined within the cell. The
is brought about by the 'cold' combustion of small pure lead grids used in the construction minimize the
quantities of gas present, and occurs to a certain extent evolution of hydrogen on overcharge. Although most
at the negative plate. of the hydrogen is recombined within the cell, some is
released to the atmosphere.
Oxygen will react with lead at the negative plate
18.2.3 Immobilization of electrolyte in the presence of sulphuric acid as quickly as it can
diffuse to the lead surface. Hydrogen will be oxidized
One of the design features of most of the available at the lead dioxide surface of the positive plate at
types of partially recombining lead-acid batteries is a somewhat lower rate, as shown by the following
that the sulphuric acid electrolyte is immobilized with overcharge recombination reactions:
thixotropic silicic acid. A constant regeneration of the
thixotropic gel structure takes place through changes Pb + HS04 + H+ + $02 + PbS04 + HzO
in volumes and the formation of minute bubbles of gas
in the pores of the electrode. This gives rise to a gentle PbOz + HS04 + Hf + Hz + PbSO4 + 2Hz0
mechanical movement of the gel, thereby avoiding loss
of contact between the electrolyte and the plates. The
thixotropically immobilized 'dry' electrolyte allows Terminal Potting material
(black = negative,
the electrolysis gases to contact the negative plate at Inner cover 1 Outer cover red = positive)
which oxygen recombination occurs, thereby produc- (sonic welded)
ing a reduction in gas pressure. The electrolyte used Potting 0.040 inch
in batteries obtainable from certain suppliers, e.g. Son- fillet vent hole
nenschein Dryfit batteries, also contains phosphoric &
acid additives to improve the constancy of recover- Pressure
able charge during battery life. Lead-acid batteries are relief /
characterized by the presence of the relatively large valve/
volume of free electrolyte or, in the case of partially without
recombining batteries, of the gel. This is in contrast to ring seal
nickel-cadmium batteries, in which there is a much Absorbent
smaller amount of potassium hydroxide electrolyte, separator
used to
which does not change greatly in volume during charge immobilize
and discharge. In the alkaline battery the porosity of electrolyte
the electrodes and the fleecy plastics separator (com-
monly Nylon) absorb and consequently immobilize
most of the electrolyte. In alkaline button cells, the
formation of damaging interstices is prevented by a
spring mechanism, which compresses the group of
plates. This spring simultaneously provides contact and
a space for gas at the negative electrode.
Calcium-lead alloy batteries, because of the gelled
electrolyte, are insensitive to orientation, as are Cell case Semipermeable
(high impact
sealed nickel-cadmium cells. They can therefore polystyrene) membrane
be stored, charged or discharged in any position, separator
even upside-down. This characteristic has obvious Figure 18.3 Eagle Picher Carefree maintenance-free lead-acid
advantages in cyclic or float applications such battery construction (Courtesy of Eagle Picher)