Page 273 - Battery Reference Book
P. 273
Mercury-zinc primary batteries 23/3
23.1 Mercury-zinc primary batteries electrolyte is 9- 10~ potassium hydroxide containing a
low concentration of zinc oxide to reduce zinc gassing.
The overall cell reaction occurring in a mercuric Mercury batteries and cells are produced in cylin-
oxide-zinc cell is: drical and button types. Electrochemically, both are
HgO + Zn = ZnO + Wg (cell voltage 1.343 V) (23.1) identical and they differ only in can design and internal
arrangement. The anode is formed from cylinders or
Up-to-date information on the construction of a pellets or powdered high-purity amalgamated zinc, or
mercuric oxide primary button cell is discussed below. a gelled mixture of electrolyte and zinc. The depolariz-
Button cells with solid cathodes are designed as ing cathode is compressed mercuric oxide-manganese
anode limited 1:i.e. 5% more cathode capacity than dioxide in sleeve or pellet form (cell voltage 1.4V)
anode capacity). The anode is amalgamated pow- or pure mercuric oxide (cell voltage 1.35 V), and the
dered or gelled zinc and contains a gelling agent, electrolyte, which does not participate in the reaction,
and is housed in a triclad metal top (nickel-stainless is concentrated aqueous sodium or potassium hydrox-
steel-copper) with the copper in direct contact with the ide. The cathode is separated from the anode by an
zinc. The anode is separated from the mercuric oxide ion-permeable barrier. In operation this combination
cathode by a separator or absorbent material (cotton- produces metallic mercury, which does not inhibit the
like material) and a semi-permeable membrane barrier. current flow within the cell (Figure 23.1). Zinc is ther-
The design of the absorbentkeparator system modynamically unstable with respect to water and in
depends on the intended use of the cell. An im- strongly alkaline solutions will tend to self-discharge
portant application is hearing aid cells. These utilize with the evolution of hydrogen. Zinc anode corrosion
a mercuric oxide cathode containing graphite for is reduced to acceptable levels by amalgamating the
conductivity and a small amount of manganese dioxide zinc powder with mercury, which increases the hydro-
to disperse imbibed mercury beads upon cell discharge. gen overpotential of the zinc and by ensuring that the
The cathode is pelleted and contained in a nickel plated surface area of the porous electrodes is not too high.
steel can. The cathode (positive) and anode (negative) Also it is necessary to use only materials of high hydro-
are separated by a grommet seal of Nylon 66. The gen overpotential (silver, lead, tin and copper, not iron
h
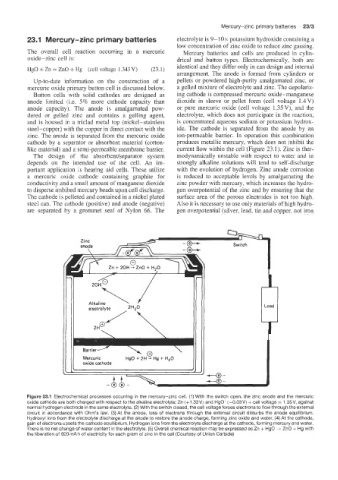
Figure 23.1 Electrochemical processes occurring in the mercury-zinc cell. (1) With the switch open, the zinc anode and the mercuric
oxide cathode are both charged with respect to the alkaline electrolyte: Zn (+I .32V) and HgO- (-0.03V) + cell voltage = 1.35V, against
normal hydrogen electrode in the same electrolyte. (2) With the switch closed, the cell voltage forces electrons to flow through the external
circuit in accordance with Ohm’s iaw. (3) At the anode, loss of electrons through the external circuit disturbs the anode equilibrium.
Hydroxyl ions from the electrolyte discharge at the anode to restore the anode charge, forming zinc oxide and water. (4) At the cathode,
gain of electrons upsets the cathode equilibrium. Hydrogen ions from the electrolyte discharge at the cathode, forming mercury and water.
There is no net change of water content in the electrolyte. (5) Overall chemical reaction may be expressed as Zn + HgO + ZnO + Hg with
the liberation of 820 mA h of electricity for each gram of zinc in the cell (Courtesy of Union Carbide)