Page 269 - Battery Reference Book
P. 269
Carbon-zinc chloride primary batteries 22/13
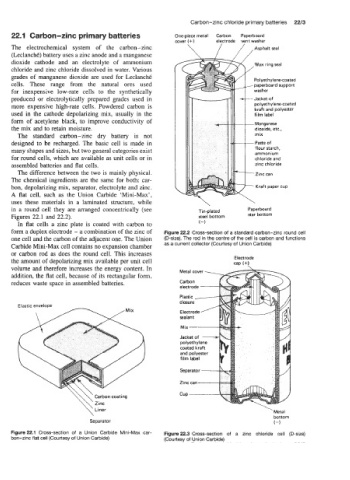
22.1 Carbon-zinc primary batteries One-piece metal Carbon Paperboard
wver (+I electrode vent washer
The electrochemical system of the carbon-zinc. \ 1 ,/ ,Asphalt Seal
(LeclanchC) battery uses a zinc anode and a manganese I //
dioxide cathode and an electrolyte of ammonium ,Wax ring Seal
chloride and zinc chloride dissolved in water. Various
grades of manganese dioxide are used for Leclanch6
cells. These range from the natural ores used Polyethylene-coated
paperboard support
for inexpensive low-rate cells to the synthetically washer
produced or electrolytically prepared grades used in Jacket of
more expensive high-rate cells. Powdered carbon is polyethylene-coated
used in the cathode depolarizing mix, usually in the
form of acetylene black, to improve conductivity of
the mix and to retain moisture.
The standard carbon-zinc dry battery is not
designed to be recharged. The basic cell is made in
many shapes and sizes, but two general categories exist ammonium
for round cells, which are available as unit cells or in chloride and
assembled batteries and flat cells.
The difference between the two is mainly physical.
The chemical ingredients are the same for both: car-
bon, depolarizing mix, separator, electrolyte and zinc.
A flat cell, such as the Union Carbide ‘Mini-Max’,
uses these materials in a laminated structure, while
in a round cell they are arranged concentrically (see Tin-plated Paperboard
Figures 22.1 and 22.2). steel bottom star bottom
In flat cells a zinc plate is coated with carbon to (-)
form a duplex electrode - a combination of the zinc of Figure 22.2 Cross-section of a standard carbon-zinc round cell
one cell and the carbon of the adjacent one. The Union (0-size). The rod in the centre of the cell is carbon and functions
Carbide Mini-Max cell contains no expansion chamber as a current collector (Courtesy of Union Carbide)
or carbon rod as does the round cell. This increases
Electrode
the amount of depolarizing mix available per unit cell cap (+\
volume and therefore increases the energy content. In
addition, the flat cell, because of its rectangular form,
reduces waste space in assembled batteries.
Elastic envelope
n ; + ( $ Y coating
Liner \
Metal
bottom
Separator (-)
Figure22.1 Cross-section of a Union Carbide Mini-Max car- Figure22.3 Cross-section of a zinc chloride cell (D-size)
bon-zinc flat cell (Courtesy of Union Carbide) (Courtesy of Union Carbide)