Page 265 - Battery Reference Book
P. 265
Alkaline manganese secondary batteries 2113
21.1 Alkaline manganese primary powder inserted. Contact to the negative terminal at
batteries the base of the battery is by an internal metal contact.
This design gives a high current density per unit vol-
Cylindrical alkaline manganese batteries have an ume of active materials, low resistance and impedance,
inverted construction compared with the familiar high service capacity, and a relatively constant capa-
LeclanchC carbon-zinc battery construction. A typical city over a range of current drawing and discharge
construction of this type of battery is shown in schedules. Cylindrical alkaline manganese cells are
Figure 21.1. fitted with an outer steel jacket which is electrically
The basic cell reaction is: isolated from the cell can. This type of cell differs
from the carbon-zinc type primarily in that a strongly
Zn(s) + MnOz(s) + f HzO (1) + MnO(OH)(s) + ZnO (s) alkaline electrolyte is used instead of the acidic am-
(21.1) monium chloride or zinc chloride medium used in the
carbon-zinc design.
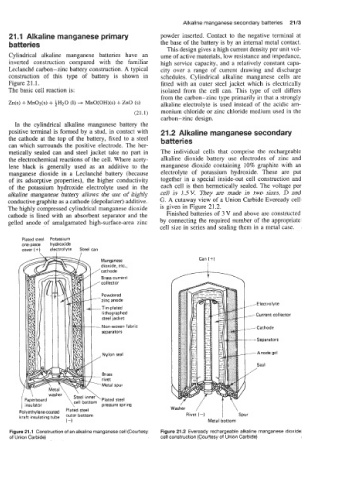
In the cylindrical alkaline manganese battery the
positive terminal is formed by a stud, in contact with 21.2 Alkaline manganese secondary
the cathode at the top of the battery, fixed to a steel batteries
can which surrounds the positive electrode. The her-
metically sealed can and steel jacket take no part in The individual cells that comprise the rechargeable
the electrochemical reactions of the cell. Where acety- alkaline dioxide battery use electrodes of zinc and
lene black is generally used as an additive to the manganese dioxide containing 10% graphite with an
manganese dioxide in a LeclanchC battery (because electrolyte of potassium hydroxide. These are put
of its adsorptive properties), the higher conductivity together in a special inside-out cell construction and
of the potassium hydroxide electrolyte used in the each cell is then hermetically sealed. The voltage per
alkaline manganese battery allows the use of highly cell is 1.5V. They are made in two sizes, D and
conductive graphite as a cathode (depolarizer) additive. G. A cutaway view of a Union Carbide Eveready cell
The highly compressed cylindrical manganese dioxide is given in Figure 21.2.
cathode is lined with an absorbent separator and the Finished batteries of 3 V and above are constructed
gelled anode of amalgamated high-surface-area zinc by connecting the required number of the appropriate
cell size in series and sealing them in a metal case.
Plated steel Potassium
one.piece hydroxide
cover (+) electrolyte Steel can
Can (+)
Current collector
Paperboard cell
insu I at or Dressure spring Washer / I
lyethylenecoated ‘Iated steel
aft insulating tube Outer bottom Rivet (-) ‘Spur
(-1 Metal bottom
Figure 21.1 Construction of an alkaline manganese cell (Courtesy Figure 21.2 Eveready rechargeable alkaline manganese dioxide
of Union Carbide) cell construction (Courtesy of Union Carbide)