Page 260 - Battery Reference Book
P. 260
2014 Sitver batteries
steel top, creating the initial radial seal. This operation Divalent silver oxide-zinc
is possible because a stainless steel anode top can
withstand the extreme pressure during the diameter Again, in the button cell designs the solid cathodes
reducing operation. Secondly, the selection of nylon are anode limited with 5-10% more cathode capacity
is important because nylon, after being squeezed, tries than anode capacity. The anode is amalgamated pow-
to retain its original position, Le. it has a memory. dered or gelled zinc and contains a gelling agent
Once the can diameter is reduced, a secondary seal and is housed in a triclad metal top (nickel-stainless
is effected by crimping the edge of the can over the steel-copper) with the copper in direct contact with
gasket. Again the use of a nylon gasket is significant zinc. The anode is separated from the divalent sil-
since nylon will continue to exert pressure as a result of ver oxide cathode by a separator or absorbent material
the second sealing operation. The radial seal technique (cotton-like material) and a semi-permeable membrane
is highly effective in providing excellent protection barrier. The design of the absorbent separator sys-
against the incidence of salting. tem depends on the chemistry of the cathode and
Further details on the construction of the latest intended use.
designs of monovalent silver oxide-zinc (Ag,O) and The cathode is pelleted and contained in a nickel
divalent silver oxide (Ago)-zinc button cells are plated steel can. The cathode (positive) and anode
given below. (negative) are separated by a grommet seal of Nylon
66. The electrolyte is 9- 10 N potassium hydroxide.
Monovalent silver oxide-zinc 20.1.1 Remotely activated silver oxide-zinc
batteries
Button cells with solid cathodes are designed as anode
limited (5-10% more cathode capacity than anode
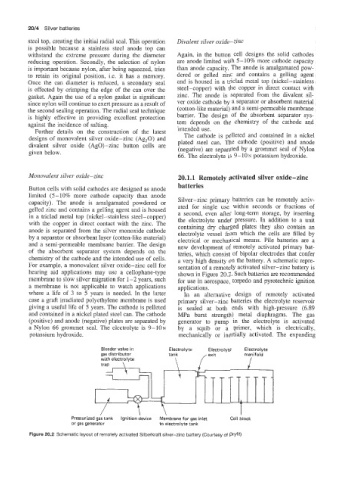
capacity). The anode is amalgamated powdered or Silver-zinc primary batteries can be remotely activ-
gelled zinc and contains a gelling agent and is housed ated for single use within seconds or fractions of
a second, even after long-term storage, by inserting
in a triclad metal top (nickel-stainless steel-copper) the electrolyte under pressure. In addition to a unit
with the copper in direct contact with the zinc. The containing dry charged plates they also contain an
anode is separated from the silver monoxide cathode electrolyte vessel from which the cells are filled by
by a separator or absorbent layer (cotten-like material) electrical or mechanical means. Pile batteries are a
and a semi-permeable membrane banier. The design new development of remotely activated primary bat-
of the absorbent separator system depends on the teries, which consist of bipolar electrodes that confer
chemistry of the cathode and the intended use of cells. a very high density on the battery. A schematic repre-
For example, a monovalent silver oxide-zinc cell for sentation of a remotely activated silver-zinc battery is
hearing aid applications may use a cellophane-type shown in Figure 20.2. Such batteries are recommended
membrane to slow silver migration for 1-2 years, such for use in aerospace, torpedo and pyrotechnic ignition
a membrane is not applicable to watch applications applications.
where a life of 3 to 5 years is needed. In the latter In an alternative design of remotely activated
case a graft irradiated polyethylene membrane is used primary silver-zinc batteries the electrolyte reservoir
giving a useful life of 5 years. The cathode is pelleted is sealed at both ends with high-pressure (6.89
and contained in a nickel plated steel can. The cathode MPa burst strength) metal diaphragms. The gas
(positive) and anode (negative) plates are separated by generator to pump in the electrolyte is activated
a Nylon 66 grommet seal, The electrolyte is 9-10~ by a squib or a primer, which is electrically,
potassium hydroxide. mechanically or inertially activated. The expanding
Bleeder valve in Electrolyte Electrolyte Electrolyte
gas distributor tank manifold
with electrolyte \ /exit I
trap \ --%
A\
7
Pressurized gas tank Ignition device Membrane for gas inlet Cell block
or gas generator to electrolyte tank
Figure 20.2 Schematic layout of remotely activated Silberkraft silver-zinc battery (Courtesy of Dryfit)