Page 259 - Battery Reference Book
P. 259
Silver oxide-zinc primary batteries 2013
20.1 Silver oxide-zinc primary seal. Mixtures of silver oxide and manganese dioxide
batteries may be tailored to provide a flat discharge curve or
increased service hours. The separator in the silver
The are two tylpes of silver oxide cell: one has a oxide system must retain silver soluble species pro-
cathode of monovalent silver oxide (Ag20) and the duced by chemical dissolution of the oxide, and a mul-
other type uses divalent silver oxide (Ago). The latter tiple layer separator of low-porosity film achieves this.
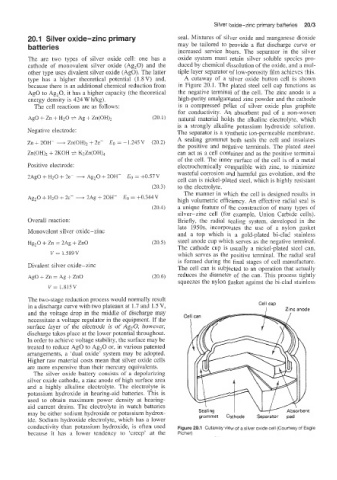
type has a highier theoretical potential (1.8 V) and, A cutaway of a silver oxide button cell is shown
because there is an additional chemical reduction from in Figure 20.1. The plated steel cell cap functions as
Ago to Ag,O. it has a higher capacity (the theoretical the negative terminal of the cell. The zinc anode is a
energy density is 424 W fig). high-purity amalgamated zinc powder and the cathode
The cell reactions are as follows: is a compressed pellet of silver oxide plus graphite
for conductivity. An absorbent pad of a non-woven
AgO + Zn + H20 + Ag + Zn(OH)2 (20.1) natural material holds the alkaline eiectrolyte, which
is a strongly alkaline potassium hydroxide solution.
Negative electrode: The separator is a synthetic ion-permeable membrane.
Zn + 2OK + &(OH), + 2e- Eo = -1.245V (20.2) A sealing grommet both seals the cell and insulates
the positive and negative terminals. The plated steel
Zn(OH), + 2KOH + KZZn(OH), can act as a cell container and as the positive terminal
of the cell. The inner surface of the cell is of a metal
Positive electrode: electrochemically compatible with zinc, to minimize
2Ag0 + H20 + 2e- + Ag,O + 2OH- Eo = +OS7 V wasteful corrosion and harmful gas evolution, and the
cell can is nickel-plated steel, which is highly resistant
(20.3) to the electrolyte.
The manner in which the cell is designed results in
AgzO + IH20 + 2t- + 2Ag + 20H- Eo = +0.344V
high volumetric efficiency. An effective radial seal is
(20.4) a unique feature of the construction of many types of
silver-zinc cell (for example, Union Carbide cells).
Overall reaction: Briefly, the radial sealing system, developed in :he
late 1950s, incorporates the use of a nylon gasket
Monovalent silver oxide-zinc
and a top which is a gold-plated bi-clad stainless
NgzO + Zn = 2Ag + ZnO (20.5) steel anode cup which serves as the negative terminal.
The cathode cup is usually a nickel-plated steel can.
v = 1.589V which serves as the positive terminal. The radial seal
is formed during the final stages of cell manufacture.
Divalent silver oxide-zinc The cell can is subjected to an operation that actually
AgO + Zn = Ag i- ZnO (20.6) reduces the diameter of the can. This process tightly
squeezes the nylon gasket against the bi-clad stainless
V = 1.815V
The two-stage reduction process would normally result
in a discharge curve with two plateaux at 1.7 and 1.5 V, Cell cap Zinc anode
I
and the voltage drop in the middle of discharge may
necessitate a voltage regulator in the equipment. If the
surface Iayer of the electrode is of Ag20, however,
discharge takes place at the lower potential throughout.
In order to achieve voltage stability, the surface may be
treated to reduce Ago to Ag20 or, in various patented
arrangements, a ‘dual oxide’ system may be adopted.
Higher raw material costs mean that silver oxide cells
are more expensive than their mercury equivalents.
The silver oxide battery consists of a depolarizing
silver oxide cathode, a zinc anode of high surface area
and a highly alkaline electrolyte. The electrolyte is
potassium hydroxide in hearing-aid batteries. This is
used to obtain maximum power density at hearing-
aid current drains. The electrolyte in watch batteries
may be either sodium hydroxide or potassium hydrox- Sealing
ide. Sodium hydroxide electrolyte, which has a lower grommet Cathode Separator pad
conductivity than potassium hydroxide, is often used Figure 20.1 Cutaway view of a silver oxide cell (Courtesy of Eagle
because it has a lower tendency to ’creep’ at the Picher)