Page 300 - Battery Reference Book
P. 300
Zinc-air primary batteries 26/3
Zinc-air primary batteries source. Cells have been designed with a structure
that contains two cathodes (air electrodes) in parallel
The chemical reactions occuning in a zinc-air cell are and one anode (Figure 26.l(a)). The two cathodes are
as follows: cemented to a frame to form the cell container. With air
Cathode 02 + :2Hz0 + 4e- = 40H- (26.1) cells, it is not necessary to incorporate a depolarizer,
such as manganese dioxide, into the cell. Instead, oxy-
Anode Zn + 40H- + Zn (OH):- (26.2) gen from the air is used as the depolarizer. The replace-
able element in the cell consists of a highly porous
Net reaction Zn + 02 + 2H20 + 2e- = Zn (OH);- (26.3) zinc anode, containing the proper amount of potassium
The theoretical voltage of this cell is 1.64V. Most hydroxide in dry form and wrapped with appropriate
practical cells have an open circuit voltage range separator materials. When a cell is ready to be activ-
between 1.42 and 1.52V, the lower values being ated, the cell container is filled with water. The zinc
atributed to peroxide build-up at the electrode surface. anode with the dry potassium hydroxide and separator
Although zinc-air batteries have been in use for is then inserted into the cell. The cell is now ready for
many years, their use has been restricted to very low- use. Multi-cell batteries can be constructed. Spacers
rate applications. As a result of the work performed on are placed between cells to permit air flow by convec-
fuel cells and advancements in technology for making tion. For heavy loads and large batteries, convection
thin gas electrodes that operate at high current dens- may not suffice and blowers are required. When spent,
ities. zinc-sir cells are now being examined as a power the zinc anodes are replaced with new ones for another
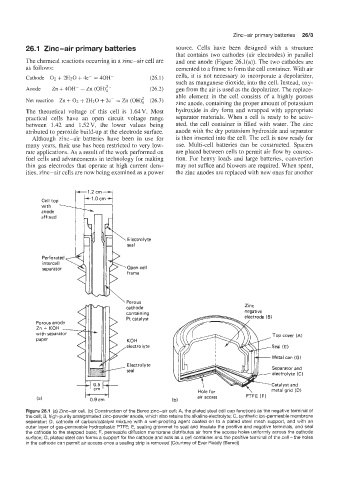
Cell top
with
anode
ib
affixed
Electroll e
seal
Perforated
intercell
separator Open cell
frame
\Porous
cathode Zinc
containing negative
Pt catalyst
Porous anode
ZnfKOH .-
with separator Top cover (A)
paper KOH
electrolyte Seal (E)
Metal can (G)
Electrolyte Separator and
seal
electrolyte (C)
Catalyst and
metal grid (C)
air access PTFE (F)
(a1 (b)
Figure 26.1 (a) Zinc-air cell. (b) Construction of the Berec zinc-air cell: A, the plated steel cell cap functions as the negative terminai of
the cell; B, high-purity amalgamated zinc-powder anode, which also retains the alkaline electrolyte; C, synthetic ion-permeable membrane
separator; D, cathode of carbonkatalyst mixture with a wet-proofing agent coated on to a plated steel mesh support, and with an
outer layer of gas-permeable hydroplastic PTFE; E, sealing grommet to seal and insulate the positive and negative terminals, and seal
the cathode to the stepped base; F, permeable diffusion membrane distributes air from the access holes uniformly across the cathode
surface; G, plated steel can forms a support for the cathode and acts as a cell container and the positive terminal of the cell -the holes
in the cathode cai permit air access once a sealing strip is removed (Courtesy of Ever Ready (Berec))