Page 33 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 33
1.4 Directed Evolution of Laccases 9
laccase mutant obtained (the ChU-B variant) was comprehensively characterized
and tested in real human blood samples, revealing the mechanisms underlying
this unprecedented improvement. The ChU-B variant conserved a high-redox
potential at the T1 site and exhibited the highest tolerance to halides reported for
−
any HRPL (with an increase in the I 50 for Cl from 176 mM to over 1 M with
′
-2,2 -azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) as the substrate), and
it displayed significant activity at neutral pH (retaining 50% and 20% of its
activity for 2,6-dimethoxyphenol (DMP) and ABTS, respectively). This was the first
successful example of the use of laboratory evolution to optimize an oxidoreductase
for enhanced catalysis in blood for biomedical purposes. From a more general
point of view, this development is of considerable importance for a wide range
of biotechnological sectors (e.g., bioremediation, pulp-kraft biobleaching), and
especially in biocatalysis to develop novel green syntheses. With respect to the
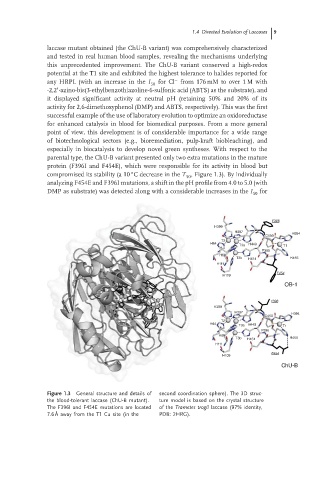
parental type, the ChU-B variant presented only two extra mutations in the mature
protein (F396I and F454E), which were responsible for its activity in blood but
◦
compromised its stability (a 10 C decrease in the T , Figure 1.3). By individually
50
analyzing F454E and F396I mutations, a shift in the pH profile from 4.0 to 5.0 (with
DMP as substrate) was detected along with a considerable increases in the I for
50
F396
H399
H397 H394
C450
T2
H64 H449
T3a T1
P395
H66
T3b H451 H455
H111
F454
H109
OB-1
1396
H399
H397 H394
C450
T2
H64 T3a H449 T1
P395
H66
T3b H451 H455
H111
E454
H109
ChU-B
Figure 1.3 General structure and details of second coordination sphere). The 3D struc-
the blood-tolerant laccase (ChU-B mutant). ture model is based on the crystal structure
The F396I and F454E mutations are located of the Trametes trogii laccase (97% identity,
7.6 ˚ A away from the T1 Cu site (in the PDB: 2HRG).