Page 307 - Biodegradable Polyesters
P. 307
11.3 Uniquely Encapsulated Drug/Biopolymer Nanofiber Systems for Drug Delivery 285
To syringe pump
To syringe pump
Cationized Network of
gelatin Anionic cationized
solution bioactive gelatin
PCL agent
solution
Cationized gelatin
Coaxial V
spinneret
Robust PCL
Cationized
gelatin
hydrogel
Fibrous membrane Core-shell fiber
Coaxial electrospinning
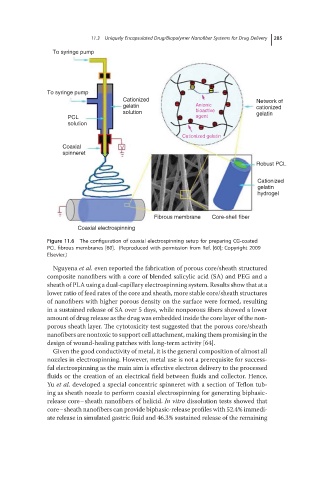
Figure 11.6 The configuration of coaxial electrospinning setup for preparing CG-coated
PCL fibrous membranes [60]. (Reproduced with permission from Ref. [60]; Copyright 2009
Elsevier.)
Nguyena et al. even reported the fabrication of porous core/sheath structured
composite nanofibers with a core of blended salicylic acid (SA) and PEG and a
sheath of PLA using a dual-capillary electrospinning system. Results show that at a
lower ratio of feed rates of the core and sheath, more stable core/sheath structures
of nanofibers with higher porous density on the surface were formed, resulting
in a sustained release of SA over 5 days, while nonporous fibers showed a lower
amount of drug release as the drug was embedded inside the core layer of the non-
porous sheath layer. The cytotoxicity test suggested that the porous core/sheath
nanofibers are nontoxic to support cell attachment, making them promising in the
design of wound-healing patches with long-term activity [64].
Given the good conductivity of metal, it is the general composition of almost all
nozzles in electrospinning. However, metal use is not a prerequisite for success-
ful electrospinning as the main aim is effective electron delivery to the processed
fluids or the creation of an electrical field between fluids and collector. Hence,
Yu et al. developed a special concentric spinneret with a section of Teflon tub-
ing as sheath nozzle to perform coaxial electrospinning for generating biphasic-
release core–sheath nanofibers of helicid. In vitro dissolution tests showed that
core–sheath nanofibers can provide biphasic-release profiles with 52.4% immedi-
ate release in simulated gastric fluid and 46.3% sustained release of the remaining