Page 24 - Biofuels Refining and Performance
P. 24
Energy and Its Biological Resources 7
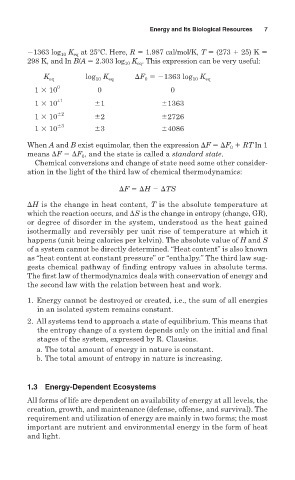
1363 log K at 25 C. Here, R 1.987 cal/mol/K, T (273 25) K
10
eq
298 K, and ln B/A 2.303 log K . This expression can be very useful:
eq
10
K eq log 10 K eq F 1363 log 10 K eq
0
1 10 0 0 0
1 10 ±1 1 1363
1 10 2 2 2726
1 10 3 3 4086
When A and B exist equimolar, then the expression F F RT ln 1
0
means F F , and the state is called a standard state.
0
Chemical conversions and change of state need some other consider-
ation in the light of the third law of chemical thermodynamics:
F H TS
H is the change in heat content, T is the absolute temperature at
which the reaction occurs, and S is the change in entropy (change, GR),
or degree of disorder in the system, understood as the heat gained
isothermally and reversibly per unit rise of temperature at which it
happens (unit being calories per kelvin). The absolute value of H and S
of a system cannot be directly determined. “Heat content” is also known
as “heat content at constant pressure” or “enthalpy.” The third law sug-
gests chemical pathway of finding entropy values in absolute terms.
The first law of thermodynamics deals with conservation of energy and
the second law with the relation between heat and work.
1. Energy cannot be destroyed or created, i.e., the sum of all energies
in an isolated system remains constant.
2. All systems tend to approach a state of equilibrium. This means that
the entropy change of a system depends only on the initial and final
stages of the system, expressed by R. Clausius.
a. The total amount of energy in nature is constant.
b. The total amount of entropy in nature is increasing.
1.3 Energy-Dependent Ecosystems
All forms of life are dependent on availability of energy at all levels, the
creation, growth, and maintenance (defense, offense, and survival). The
requirement and utilization of energy are mainly in two forms; the most
important are nutrient and environmental energy in the form of heat
and light.