Page 95 - Biofuels Refining and Performance
P. 95
78 Chapter Three
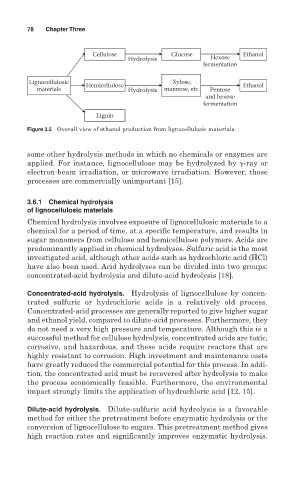
Cellulose Glucose Ethanol
Hydrolysis Hexose
fermentation
Lignocellulosic Xylose,
Hemicellulose Ethanol
materials Hydrolysis mannose, etc. Pentose
and hexose
fermentation
Lignin
Figure 3.5 Overall view of ethanol production from lignocellulosic materials.
some other hydrolysis methods in which no chemicals or enzymes are
applied. For instance, lignocellulose may be hydrolyzed by -ray or
electron-beam irradiation, or microwave irradiation. However, those
processes are commercially unimportant [15].
3.6.1 Chemical hydrolysis
of lignocellulosic materials
Chemical hydrolysis involves exposure of lignocellulosic materials to a
chemical for a period of time, at a specific temperature, and results in
sugar monomers from cellulose and hemicellulose polymers. Acids are
predominantly applied in chemical hydrolyses. Sulfuric acid is the most
investigated acid, although other acids such as hydrochloric acid (HCl)
have also been used. Acid hydrolyses can be divided into two groups:
concentrated-acid hydrolysis and dilute-acid hydrolysis [18].
Concentrated-acid hydrolysis. Hydrolysis of lignocellulose by concen-
trated sulfuric or hydrochloric acids is a relatively old process.
Concentrated-acid processes are generally reported to give higher sugar
and ethanol yield, compared to dilute-acid processes. Furthermore, they
do not need a very high pressure and temperature. Although this is a
successful method for cellulose hydrolysis, concentrated acids are toxic,
corrosive, and hazardous, and these acids require reactors that are
highly resistant to corrosion. High investment and maintenance costs
have greatly reduced the commercial potential for this process. In addi-
tion, the concentrated acid must be recovered after hydrolysis to make
the process economically feasible. Furthermore, the environmental
impact strongly limits the application of hydrochloric acid [12, 15].
Dilute-acid hydrolysis. Dilute-sulfuric acid hydrolysis is a favorable
method for either the pretreatment before enzymatic hydrolysis or the
conversion of lignocellulose to sugars. This pretreatment method gives
high reaction rates and significantly improves enzymatic hydrolysis.