Page 224 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 224
Chapter | 7 Gasification Theory 201
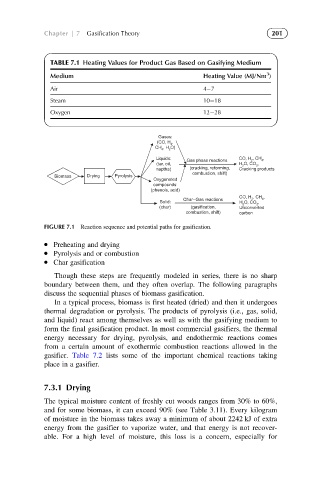
TABLE 7.1 Heating Values for Product Gas Based on Gasifying Medium
3
Medium Heating Value (MJ/Nm )
Air 4 7
Steam 10 18
Oxygen 12 28
Gases:
(CO, H ,
2
, H O)
CH 4
2
Liquids: Gas phase reactions CO, H 2 , CH , 4
(tar, oil, H O, CO , 2
2
naptha) (cracking, reforming, Cracking products
combustion, shift)
Biomass Drying Pyrolysis
Oxygenated
compounds:
(phenols, acid)
, CH ,
Char–Gas reactions 4
CO, H 2
Solid: H O, CO ,
2
2
(char) (gasification, Unconverted
combustion, shift) carbon
FIGURE 7.1 Reaction sequence and potential paths for gasification.
Preheating and drying
Pyrolysis and or combustion
Char gasification
Though these steps are frequently modeled in series, there is no sharp
boundary between them, and they often overlap. The following paragraphs
discuss the sequential phases of biomass gasification.
In a typical process, biomass is first heated (dried) and then it undergoes
thermal degradation or pyrolysis. The products of pyrolysis (i.e., gas, solid,
and liquid) react among themselves as well as with the gasifying medium to
form the final gasification product. In most commercial gasifiers, the thermal
energy necessary for drying, pyrolysis, and endothermic reactions comes
from a certain amount of exothermic combustion reactions allowed in the
gasifier. Table 7.2 lists some of the important chemical reactions taking
place in a gasifier.
7.3.1 Drying
The typical moisture content of freshly cut woods ranges from 30% to 60%,
and for some biomass, it can exceed 90% (see Table 3.11). Every kilogram
of moisture in the biomass takes away a minimum of about 2242 kJ of extra
energy from the gasifier to vaporize water, and that energy is not recover-
able. For a high level of moisture, this loss is a concern, especially for