Page 227 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 227
204 Biomass Gasification, Pyrolysis and Torrefaction
10
0.1 Mpa, covered crucible;
9
k=0.013 L/min and k1=0.56
Reactivity in steam (%/min) 6 5 4 3
8
0.73 Mpa; k=0.067 L/min
7
and k1=0.76
1 2
0
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
Char conversion (-)
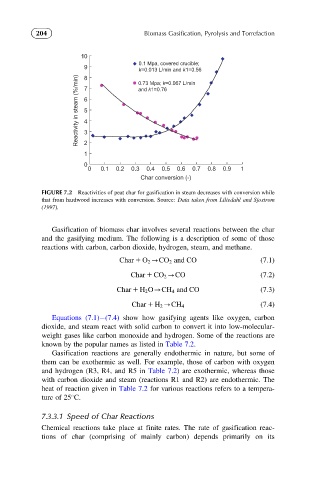
FIGURE 7.2 Reactivities of peat char for gasification in steam decreases with conversion while
that from hardwood increases with conversion. Source: Data taken from Liliedahl and Sjostrom
(1997).
Gasification of biomass char involves several reactions between the char
and the gasifying medium. The following is a description of some of those
reactions with carbon, carbon dioxide, hydrogen, steam, and methane.
Char 1 O 2 -CO 2 and CO (7.1)
Char 1 CO 2 -CO (7.2)
Char 1 H 2 O-CH 4 and CO (7.3)
(7.4)
Char 1 H 2 -CH 4
Equations (7.1) (7.4) show how gasifying agents like oxygen, carbon
dioxide, and steam react with solid carbon to convert it into low-molecular-
weight gases like carbon monoxide and hydrogen. Some of the reactions are
known by the popular names as listed in Table 7.2.
Gasification reactions are generally endothermic in nature, but some of
them can be exothermic as well. For example, those of carbon with oxygen
and hydrogen (R3, R4, and R5 in Table 7.2) are exothermic, whereas those
with carbon dioxide and steam (reactions R1 and R2) are endothermic. The
heat of reaction given in Table 7.2 for various reactions refers to a tempera-
ture of 25 C.
7.3.3.1 Speed of Char Reactions
Chemical reactions take place at finite rates. The rate of gasification reac-
tions of char (comprising of mainly carbon) depends primarily on its