Page 230 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 230
Chapter | 7 Gasification Theory 207
The shift reaction is slightly exothermic, and its equilibrium yield
decreases slowly with temperature. Depending on temperature, it may be
driven in either direction, that is, products or reactants. However, it is not
sensitive to pressure (Petersen and Werther, 2005).
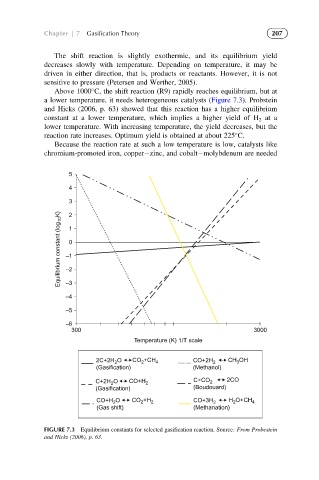
Above 1000 C, the shift reaction (R9) rapidly reaches equilibrium, but at
a lower temperature, it needs heterogeneous catalysts (Figure 7.3). Probstein
and Hicks (2006, p. 63) showed that this reaction has a higher equilibrium
constant at a lower temperature, which implies a higher yield of H 2 at a
lower temperature. With increasing temperature, the yield decreases, but the
reaction rate increases. Optimum yield is obtained at about 225 C.
Because the reaction rate at such a low temperature is low, catalysts like
chromium-promoted iron, copper zinc, and cobalt molybdenum are needed
5
4
3
Equilibrium constant (log 10 K) –1
2
1
0
–2
–3
–4
–5
–6
300 3000
Temperature (K) 1/T scale
2C+2H O CO +CH 4 CO+2H 2 CH OH
2
3
2
(Gasification) (Methanol)
C+2H O CO+H 2 C+CO 2 2CO
2
(Gasification) (Boudouard)
CO+H O CO 2 +H 2 CO+3H 2 H O+CH 4
2
2
(Gas shift) (Methanation)
FIGURE 7.3 Equilibrium constants for selected gasification reaction. Source: From Probestein
and Hicks (2006), p. 63.