Page 255 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 255
Chapter | 7 Gasification Theory 231
is entirely consumed by the reaction on the outer surface of the char, gasifi-
cation is restricted to the external surface area. This can happen because of
the limitation of the mass transfer of gas to the char surface. We can illus-
trate using the example of char gasification in CO 2 :
C 1 CO 2 -2CO (7.58)
Here, the CO 2 gas has to diffuse to the char surface to react with the
active carbon sites. The diffusion, however, takes place at a finite rate. If the
kinetic rate of this reaction is much faster than the diffusion rate of CO 2 to
the char surface, all of the CO 2 gas molecules transported are consumed
on the external surface of the char, leaving none to enter the pores and react
on their surfaces. As the overall reaction is controlled by diffusion, it is
called the diffusion- or mass-transfer-controlled regime of reaction.
On the other hand, if the kinetic rate of reaction is slow compared to the
transport rate of CO 2 molecules, then the CO 2 will diffuse into the pores and
react on their walls. The reaction in this situation is “kinetically controlled.”
Diffusion rateckinetic rate ½kinetic control reaction
(7.59)
Diffusion rate{kinetic rate ½diffusion control reaction
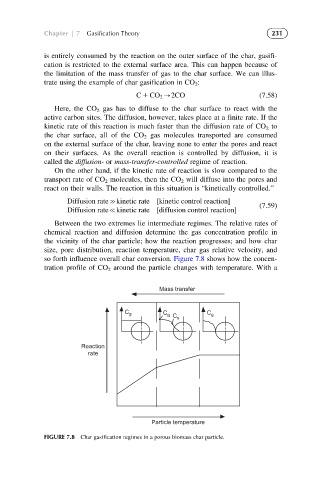
Between the two extremes lie intermediate regimes. The relative rates of
chemical reaction and diffusion determine the gas concentration profile in
the vicinity of the char particle; how the reaction progresses; and how char
size, pore distribution, reaction temperature, char gas relative velocity, and
so forth influence overall char conversion. Figure 7.8 shows how the concen-
tration profile of CO 2 around the particle changes with temperature. With a
Mass transfer
C C
C g g C s g
Reaction
rate
Particle temperature
FIGURE 7.8 Char gasification regimes in a porous biomass char particle.