Page 218 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 218
STERILE MEDICAL DEVICE PACKAGE DEVELOPMENT 197
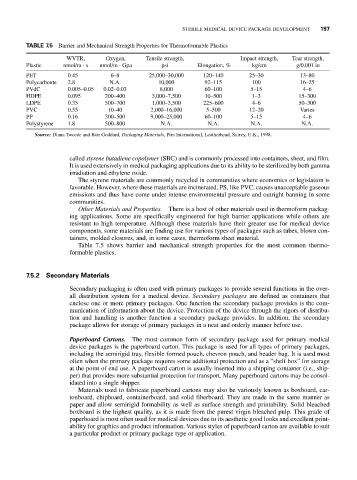
TABLE 7.5 Barrier and Mechanical Strength Properties for Thermoformable Plastics
WVTR, Oxygen, Tensile strength, Impact strength, Tear strength,
.
.
Plastic nmol/m s nmol/m Gpa psi Elongation, % kg/cm g/0.001 in
PET 0.45 6–8 25,000–30,000 120–140 25–30 13–80
Polycarbonte 2.8 N.A. 10,000 92–115 100 16–25
PVdC 0.005–0.05 0.02–0.03 8,000 60–100 5–15 4–6
HDPE 0.095 200–400 3,000–7,500 10–500 1–3 15–300
LDPE 0.35 500–700 1,000–3,500 225–600 4–6 50–300
PVC 0.55 10–40 2,000–16,000 5–500 12–20 Varies
PP 0.16 300–500 9,000–25,000 60–100 5–15 4–6
Polystyrene 1.8 500–800 N.A. N.A. N.A. N.A.
Source: Diana Tweede and Ron Goddard, Packaging Materials, Pira International, Leatherhead, Surrey, U.K., 1998.
called styrene butadiene copolymer (SBC) and is commonly processed into containers, sheet, and film.
It is used extensively in medical packaging applications due to its ability to be sterilized by both gamma
irradiation and ethylene oxide.
The styrene materials are commonly recycled in communities where economics or legislation is
favorable. However, where these materials are incinerated, PS, like PVC, causes unacceptable gaseous
emissions and thus have come under intense environmental pressure and outright banning in some
communities.
Other Materials and Properties. There is a host of other materials used in thermoform packag-
ing applications. Some are specifically engineered for high barrier applications while others are
resistant to high temperature. Although these materials have their greater use for medical device
components, some materials are finding use for various types of packages such as tubes, blown con-
tainers, molded closures, and, in some cases, thermoform sheet material.
Table 7.5 shows barrier and mechanical strength properties for the most common thermo-
formable plastics.
7.5.2 Secondary Materials
Secondary packaging is often used with primary packages to provide several functions in the over-
all distribution system for a medical device. Secondary packages are defined as containers that
enclose one or more primary packages. One function the secondary package provides is the com-
munication of information about the device. Protection of the device through the rigors of distribu-
tion and handling is another function a secondary package provides. In addition, the secondary
package allows for storage of primary packages in a neat and orderly manner before use.
Paperboard Cartons. The most common form of secondary package used for primary medical
device packages is the paperboard carton. This package is used for all types of primary packages,
including the semirigid tray, flexible formed pouch, chevron pouch, and header bag. It is used most
often when the primary package requires some additional protection and as a “shelf box” for storage
at the point of end use. A paperboard carton is usually inserted into a shipping container (i.e., ship-
per) that provides more substantial protection for transport. Many paperboard cartons may be consol-
idated into a single shipper.
Materials used to fabricate paperboard cartons may also be variously known as boxboard, car-
tonboard, chipboard, containerboard, and solid fiberboard. They are made in the same manner as
paper and allow semirigid formability as well as surface strength and printability. Solid bleached
boxboard is the highest quality, as it is made from the purest virgin bleached pulp. This grade of
paperboard is most often used for medical devices due to its aesthetic good looks and excellent print-
ability for graphics and product information. Various styles of paperboard carton are available to suit
a particular product or primary package type or application.