Page 241 - Biosystems Engineering
P. 241
Biomass Pyr olysis and Bio-Oil Refineries 219
H O
HO O C=C CH-CH
O CH 3 CH + H O + CO + CO O
2
2
R O
6
Fragmentation Fragmentation
3 3 Char
1 2 O
HO
Fast Depolymerization H + .. 5
Cellulose Cellulose of low H O
DP (active cellulose) H H
H
HO HO
Alkali cation–inhibited Lignin-inhibited
mechanism mechanism
7 4
(Slow pyrolysis) Acid (phosphoric acids) + H O
water catalyzed 2 O
O
H O + CO + CO + chain linked O O CH
2
2
by ether groups
Levoglucosenone O
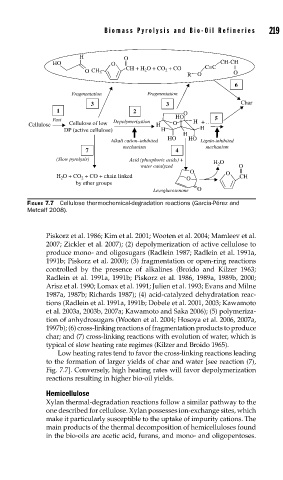
FIGURE 7.7 Cellulose thermochemical-degradation reactions (Garcia-Pérez and
Metcalf 2008).
Piskorz et al. 1986; Kim et al. 2001; Wooten et al. 2004; Mamleev et al.
2007; Zickler et al. 2007); (2) depolymerization of active cellulose to
produce mono- and oligosugars (Radlein 1987; Radlein et al. 1991a,
1991b; Piskorz et al. 2000); (3) fragmentation or open-ring reactions
controlled by the presence of alkalines (Broido and Kilzer 1963;
Radlein et al. 1991a, 1991b; Piskorz et al. 1986, 1989a, 1989b, 2000;
Arisz et al. 1990; Lomax et al. 1991; Julien et al. 1993; Evans and Milne
1987a, 1987b; Richards 1987); (4) acid-catalyzed dehydratation reac-
tions (Radlein et al. 1991a, 1991b; Dobele et al. 2001, 2003; Kawamoto
et al. 2003a, 2003b, 2007a; Kawamoto and Saka 2006); (5) polymeriza-
tion of anhydrosugars (Wooten et al. 2004; Hosoya et al. 2006, 2007a,
1997b); (6) cross-linking reactions of fragmentation products to produce
char; and (7) cross-linking reactions with evolution of water, which is
typical of slow heating rate regimes (Kilzer and Broido 1965).
Low heating rates tend to favor the cross-linking reactions leading
to the formation of larger yields of char and water [see reaction (7),
Fig. 7.7]. Conversely, high heating rates will favor depolymerization
reactions resulting in higher bio-oil yields.
Hemicellulose
Xylan thermal-degradation reactions follow a similar pathway to the
one described for cellulose. Xylan possesses ion-exchange sites, which
make it particularly susceptible to the uptake of impurity cations. The
main products of the thermal decomposition of hemicelluloses found
in the bio-oils are acetic acid, furans, and mono- and oligopentoses.