Page 395 - Biosystems Engineering
P. 395
372 Cha pte r T h i r tee n
other processes. However, the process is expensive because solvent
cannot be recovered completely.
Oil Recovery: A Novel Azeotropic Mixture for Extracting Solvents from Edible
Oils Edible oil extracted by a conventional mechanical press bears
many impurities such as free fatty acids, colored and other gummy
materials, which are known to be detrimental to oil flavor and stabil-
2
ity. Hexane is generally used as a solvent to extract oil, but a question
3
2
arises about its safety due to the presence of solvent in the oil as well
as solvent vapor, which is a hazardous air pollutant. Ethanol, metha-
nol, and acetone have been recommended as solvents for for extract-
ing vegetable oils. Nag et al. carried out oil extraction from flax and
4
bahera seeds with isopropyl alcohol, ethanol, and ethyl acetate and
have reached the following conclusions:
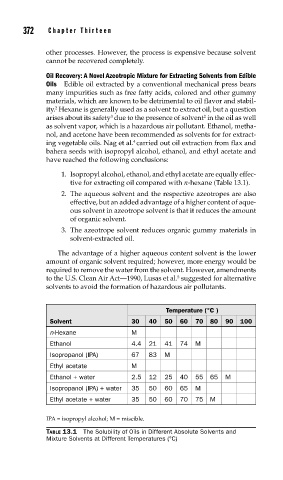
1. Isopropyl alcohol, ethanol, and ethyl acetate are equally effec-
tive for extracting oil compared with n-hexane (Table 13.1).
2. The aqueous solvent and the respective azeotropes are also
effective, but an added advantage of a higher content of aque-
ous solvent in azeotrope solvent is that it reduces the amount
of organic solvent.
3. The azeotrope solvent reduces organic gummy materials in
solvent-extracted oil.
The advantage of a higher aqueous content solvent is the lower
amount of organic solvent required; however, more energy would be
required to remove the water from the solvent. However, amendments
to the U.S. Clean Air Act—1990, Lusas et al. suggested for alternative
5
solvents to avoid the formation of hazardous air pollutants.
Temperature (°C )
Solvent 30 40 50 60 70 80 90 100
n-Hexane M
Ethanol 4.4 21 41 74 M
Isopropanol (IPA) 67 83 M
Ethyl acetate M
Ethanol + water 2.5 12 25 40 55 65 M
Isopropanol (IPA) + water 35 50 60 65 M
Ethyl acetate + water 35 50 60 70 75 M
IPA = isopropyl alcohol; M = miscible.
TABLE 13.1 The Solubility of Oils in Different Absolute Solvents and
Mixture Solvents at Different Temperatures (°C)