Page 476 - Biosystems Engineering
P. 476
448 Cha pte r S i x tee n
Supercritical fluid extraction (SFE) processes use fluids that has
appreciable solubility near the sub- or supercritical point to extract
selected components by regulating the temperature, pressure, or flow
rate. Supercritical CO is the most commonly used fluid due to its low
2
critical properties, 31.1°C, 7.38 MPa, 0.468 g/cm and other advan-
3
tages. Physical properties of solvents considered for supercritical
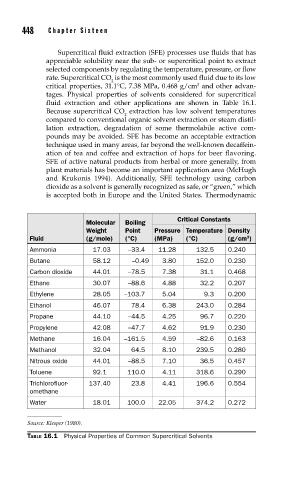
fluid extraction and other applications are shown in Table 16.1.
Because supercritical CO extraction has low solvent temperatures
2
compared to conventional organic solvent extraction or steam distil-
lation extraction, degradation of some thermolabile active com-
pounds may be avoided. SFE has become an acceptable extraction
technique used in many areas, far beyond the well-known decaffein-
ation of tea and coffee and extraction of hops for beer flavoring.
SFE of active natural products from herbal or more generally, from
plant materials has become an important application area (McHugh
and Krukonis 1994). Additionally, SFE technology using carbon
dioxide as a solvent is generally recognized as safe, or “green,” which
is accepted both in Europe and the United States. Thermodynamic
Critical Constants
Molecular Boiling
Weight Point Pressure Temperature Density
Fluid (g/mole) (°C) (MPa) (°C) (g/cm )
3
Ammonia 17.03 –33.4 11.28 132.5 0.240
Butane 58.12 –0.49 3.80 152.0 0.230
Carbon dioxide 44.01 –78.5 7.38 31.1 0.468
Ethane 30.07 –88.6 4.88 32.2 0.207
Ethylene 28.05 –103.7 5.04 9.3 0.200
Ethanol 46.07 78.4 6.38 243.0 0.284
Propane 44.10 –44.5 4.25 96.7 0.220
Propylene 42.08 –47.7 4.62 91.9 0.230
Methane 16.04 –161.5 4.59 –82.6 0.163
Methanol 32.04 64.5 8.10 239.5 0.280
Nitrous oxide 44.01 –88.5 7.10 36.5 0.457
Toluene 92.1 110.0 4.11 318.6 0.290
Trichlorofluor- 137.40 23.8 4.41 196.6 0.554
omethane
Water 18.01 100.0 22.05 374.2 0.272
Source: Klesper (1980).
TABLE 16.1 Physical Properties of Common Supercritical Solvents