Page 58 - Carbon Nanotubes
P. 58
Carbon nanotubes with single-layer walls 49
that, in that case, the nanotubes form in the gas phase. pulling the tubules out of the soot mass has aligned
Third, a soft rubbery blanket or collar builds up them to a striking degree. A high resolution TEM
around the cathode when iron group metals are used. (HRTEM) image of a group of nanotubes (Fig. 2c)
This material has been found to contain graphitic poly- demonstrates their tendency to aggregate into bundles.
hedral particles, metals or metal carbides encapsulated The aggregation process is presumably driven by van
in polyhedral particles, string of beads structures[8,16], der Waals attraction, which has been shown experi-
and single-layer nanotubes[4,8,16]. Finally, with some mentally to give rise to significant forces between ad-
catalysts, notably Co, mixtures of Co with Fe, Ni, Pt, jacent multilayer nanotubes[22], and is predicted to
S, Bi, and Pb, and Fe/Ni mixtures, web-like materi- give rise to ordering of bundled single-layer nanotubes
als form inside the chamber when the arc is running into crystalline arrays[23]. A micrograph showing a
[3,6,8,111. bundle of Ni-catalyzed nanotubes end-on lends some
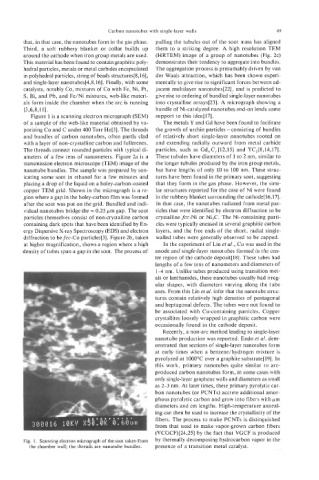
Figure 1 is a scanning electron micrograph (SEM) support to this idea[ 171.
of a sample of the web-like material obtained by va- The metals Y and Gd have been found to facilitate
porizing Co and C under 400 Torr He[3]. The threads the growth of urchin particles - consisting of bundles
and bundles of carbon nanotubes, often partly clad of relatively short single-layer nanotubes rooted on
with a layer of non-crystalline carbon and fullerenes. and extending radially outward from metal carbide
The threads connect rounded particles with typical di- particles, such as Gd,C,[12,15] and YC2[8,14,17].
ameters of a few tens of nanometers. Figure 2a is a These tubules have diameters of 1 to 2 nm, similar to
transmission electron microscope (TEM) image of the the longer tubules produced by the iron group metals,
nanotube bundles. The sample was prepared by son- but have lengths of only 10 to 100 nm. These struc-
icating some soot in ethanol for a few minutes and tures have been found in the primary soot, suggesting
placing a drop of the liquid on a holey-carbon-coated that they form in the gas phase. However, the simi-
copper TEM grid. Shown in the micrograph is a re- lar structures reported for the case of Ni were found
gion where a gap in the holey-carbon film was formed in the rubbery blanket surrounding the cathode[ 16,171.
after the soot was put on the grid. Bundled and indi- In that case, the nanotubes radiated from metal par-
vidual nanotubes bridge the = 0.25 pm gap. The soot ticles that were identified by electron diffraction to be
particles themselves consist of non-crystalline carbon crystalline fcc-Ni or Ni3C. The Ni-containing parti-
containing dark spots that have been identified by En- cles were typically encased in several graphitic carbon
ergy Dispersive X-ray Spectroscopy (EDS) and electron layers, and the free ends of the short, radial single-
diffraction to befcc-Co particles[3]. Figure 2b, taken walled tubes were generally observed to be capped.
at higher magnification, shows a region where a high In the experiment of Lin et ai., Cu was used in the
density of tubes span a gap in the soot. The process of anode and single-layer nanotubes formed in the cen-
ter region of the cathode deposit[l8]. These tubes had
lengths of a few tens of nanometers and diameters of
1-4 nm. Unlike tubes produced using transition met-
als or lanthanides, these nanotubes usually had irreg-
ular shapes, with diameters varying along the tube
axes. From this Lin et al. infer that the nanotube struc-
tures contain relatively high densities of pentagonal
and heptagonal defects. The tubes were not found to
be associated with Cu-containing particles. Copper
crystallites loosely wrapped in graphitic carbon were
occasionally found in the cathode deposit.
Recently, a non-arc method leading to single-layer
nanotube production was reported. Endo et al. dem-
onstrated that sections of single-layer nanotubes form
at early times when a benzene/hydrogen mixture is
pyrolyzed at 1000°C over a graphite substrate[l9]. In
this work, primary nanotubes quite similar to arc-
produced carbon nanotubes form, in some cases with
only single-layer graphene walls and diameters as small
as 2-3 nm. At later times, these primary pyrolytic car-
bon nanotubes (or PCNTs) accrete additional amor-
phous pyrolytic carbon and grow into fibers with pm
diameters and cm lengths. High-temperature anneal-
ing can then be used to increase the crystallinity of the
fibers. The process to make PCNTs is distinguished
from that used to make vapor-grown carbon fibers
(VCGCF)[24,25] by the fact that VGCF is produced
Fig. 1. Scanning electron micrograph of the soot taken from by thermally decomposing hydrocarbon vapor in the
the chamber wall; the threads are nanotube bundles. presence of a transition metal catalyst.