Page 67 - Carbon Nanotubes
P. 67
56 C.-H. KIANG et ai.
vapor-grown carbon fibers, but the detailed process ameter nanotubes produced with Fe, Co, and Co/S,
is not yet understood[51]. adapted from earlier reports[2,5]. In comparing the di-
ameter distributions produced using Co and Co/S,
there is striking correlation of both the overall max-
5. GROWTH OF SINGLE-LAYER ima and even the fine structures exhibited by the dis-
CARBON NANOTUBES
tributions (Figs. 5b and 5c, respectively). For the cases
There remains a major puzzle as to what controls where large diameter tubes (> 3 nm) are produced by
the growth of these nanotubes, and how it precludes adding S, Pb, or Bi to the cobalt, the tubes are still
the formation of additional layers. The reaction con- exclusively single-layered. We observed only one
ditions in the electric arc environment used for nano- double-layer nanotube out of over a thousand tubes
tube production to date are not ideal for mechanistic observed. This suggests that nucleation of additional
studies, since the plasma composition near the arc is layers must be strongly inhibited. The stability of
very complex and inhomogeneous, making individual nanotubes as a function of their diameter has been in-
variables impossible to isolate. So far, we can only ex- vestigated theoretically via classical mechanical calcu-
amine the product composition to extract clues about lations[52,53]. The tube energies vary smoothly with
the growth mechanism. One feature that can be ana- diameter, with larger diameter tubes more stable than
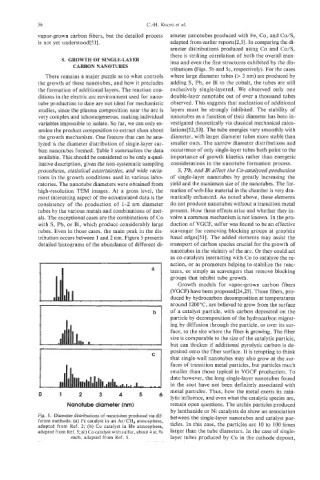
lyzed is the diameter distribution of single-layer car- smaller ones. The narrow diameter distributions and
bon nanotubes formed. Table 1 summarizes the data occurrence of only single-layer tubes both point to the
available. This should be considered to be only a qual- importance of growth kinetics rather than energetic
itative description, given the non-systematic sampling considerations in the nanotube formation process.
procedures, statistical uncertainties, and wide varia- S, Pb, and Bi affect the Co-catalyzed production
tions in the growth conditions used in various labo- of single-layer nanotubes by greatly increasing the
ratories. The nanotube diameters were obtained from yield and the maximum size of the nanotubes. The for-
high-resolution TEM images. At a gross level, the mation of web-like material in the chamber is very dra-
most interesting aspect of the accumulated data is the matically enhanced. As noted above, these elements
consistency of the production of 1-2 nm diameter do not produce nanotubes without a transition metal
tubes by the various metals and combinations of met- present. How these effects arise and whether they in-
als. The exceptional cases are the combinations of Co volve a common mechanism is not known. In the pro-
with S, Pb, or Bi, which produce considerably large duction of VGCF, sulfur was found to be an effective
tubes. Even in those cases, the main peak in the dis- scavenger for removing blocking groups at graphite
tribution occurs between 1 and 2 nm. Figure 5 presents basal edges[51]. The added elements may assist the
detailed histograms of the abundance of different di- transport of carbon species crucial for the growth of
nanotubes in the vicinity of the arc. Or they could act
as co-catalysts interacting with Co to catalyze the re-
action, or as promoters helping to stabilize the reac-
tants, or simply as scavengers that remove blocking
groups that inhibit tube growth.
Growth models for vapor-grown carbon fibers
(VGCF) have been proposed[24,25]. Those fibers, pro-
duced by hydrocarbon decomposition at temperatures
around 12OO0C, are believed to grow from the surface
of a catalyst particle, with carbon deposited on the
L
bl particle by decomposition of the hydrocarbon migrat-
ing by diffusion through the particle, or over its sur-
face, to the site where the fiber is growing. The fiber
size is comparable to the size of the catalytic particle,
c1 but can thicken if additional pyrolytic carbon is de-
posited onto the fiber surface. It is tempting to think
that single-wall nanotubes may also grow at the sur-
faces of transition metal particles, but particles much
smaller than those typical in VGCF production. To
date however, the long single-layer nanotubes found
in the soot have not been definitely associated with
metal particles. Thus, how the metal exerts its cata-
0 1 2 3 4 5 6
lytic influence, and even what the catalytic species are,
Nanotube dlameter (nm) remain open questions. The urchin particles produced
by lanthanide or Ni catalysts do show an association
Fig. 5. Diameter distributions of nanotubes produced via dif- between the single-layer nanotubes and catalyst par-
ferent methods: (a) Fe catalyst in an Ar/CH, atmosphere, ticles. In this case, the particles are 10 to 100 times
adapted from Ref. 2; (b) Co catalyst in He atmosphere,
adapted from Ref. 5; (c) Cocatalyst with sulfur, about 4 at.% larger than the tube diameters. In the case of single-
each, adapted from Ref. 5. layer tubes produced by Cu in the cathode deposit,