Page 16 - Carbonate Facies in Geologic History
P. 16
The Requisite Marine Environment: Warmth, Light, Water Movement 3
CaC0 3 . Thus, any process which removes CO 2 from normal water (pH=8.4),
tending to change bicarbonate to carbonate ions, encourages lime precipitation.
At least eight mechanisms for this process may be effective: increase of tempera-
ture, intense evaporation, influx of supersaturated water to an area where
CaC0 3 nuclei or catalyzers are present, marine upwelling from an area of high
pressure to low pressure, mixing of water high in C0 3 and low in Ca+ + with sea
water, organic processes in body fluids, bacterial decay to produce ammonia,
raising pH and increasing carbonate concentration, and removal of CO 2 by
photosynthesis.
The photosynthesis process brought about by metabolism of microplanktonic
flora, especially when operating in warm and agitated water, may be of prime
importance. If this is so-and biochemical studies indicate more and more that
organic amino acids capable of precipitating CaC0 3 coat almost all particles in
the sea (Mitterer, 1971)-important implications exist for depth control on the
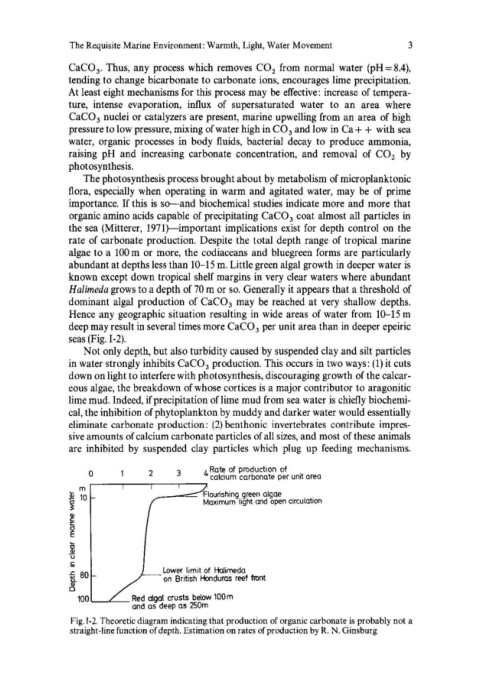
rate of carbonate production. Despite the total depth range of tropical marine
algae to a 100 m or more, the codiaceans and bluegreen forms are particularly
abundant at depths less than 10-15 m. Little green algal growth in deeper water is
known except down tropical shelf margins in very clear waters where abundant
Halimeda grows to a depth of 70 m or so. Generally it appears that a threshold of
dominant algal production of CaC0 3 may be reached at very shallow depths.
Hence any geographic situation resulting in wide areas of water from 10-15 m
deep may result in several times more CaC0 3 per unit area than in deeper epeiric
seas (Fig. 1-2).
Not only depth, but also turbidity caused by suspended clay and silt particles
in water strongly inhibits CaC0 3 production. This occurs in two ways: (1) it cuts
down on light to interfere with photosynthesis, discouraging growth of the calcar-
eous algae, the breakdown of whose cortices is a major contributor to aragonitic
lime mud. Indeed, if precipitation of lime mud from sea water is chiefly biochemi-
cal, the inhibition of phytoplankton by muddy and darker water would essentially
eliminate carbonate production: (2) benthonic invertebrates contribute impres-
sive amounts of calcium carbonate particles of all sizes, and most of these animals
are inhibited by suspended clay particles which plug up feeding mechanisms.
o 2 3 4 calcium carbonate per unit area
Rate of production of
... m r---~----'---~----'
.Sl 10 __ -==- Flourishing ,green algae, ,
Maximum light and open Circulation
~
CI>
'f
g
~
u
,I;;
.s= 80 Lower limit of Halimeda
! on British Honduras reef front
100'---L __ Red algal crusts below 100m
and as deep as 250m
Fig. 1-2. Theoretic diagram indicating that production of organic carbonate is probably not a
straight-line function of depth, Estimation on rates of production by R. N. Ginsburg