Page 209 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 209
172 Carraher’s Polymer Chemistry
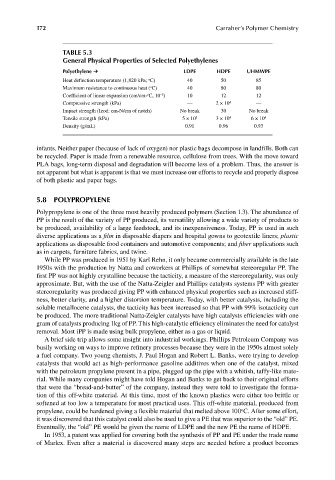
TABLE 5.3
General Physical Properties of Selected Polyethylenes
Polyethylene ➔ LDPE HDPE UHMWPE
o
Heat deflection temperature (1,820 kPa; C) 40 50 85
o
Maximum resistance to continuous heat ( C) 40 80 80
Coefficient of linear expansion (cm/cm- C, 10 ) 10 12 12
o
–5
Compressive strength (kPa) — 2 × 10 4 —
Impact strength (Izod: cm-N/cm of notch) No break 30 No break
Tensile strength (kPa) 5 × 10 3 3 × 10 4 6 × 10 4
Density (g/mL) 0.91 0.96 0.93
infants. Neither paper (because of lack of oxygen) nor plastic bags decompose in landfills. Both can
be recycled. Paper is made from a renewable resource, cellulose from trees. With the move toward
PLA bags, long-term disposal and degradation will become less of a problem. Thus, the answer is
not apparent but what is apparent is that we must increase our efforts to recycle and properly dispose
of both plastic and paper bags.
5.8 POLYPROPYLENE
Polypropylene is one of the three most heavily produced polymers (Section 1.3). The abundance of
PP is the result of the variety of PP produced, its versatility allowing a wide variety of products to
be produced, availability of a large feedstock, and its inexpensiveness. Today, PP is used in such
diverse applications as a fi lm in disposable diapers and hospital gowns to geotextile liners; plastic
applications as disposable food containers and automotive components; and fi ber applications such
as in carpets, furniture fabrics, and twine.
While PP was produced in 1951 by Karl Rehn, it only became commercially available in the late
1950s with the production by Natta and coworkers at Phillips of somewhat stereoregular PP. The
first PP was not highly crystalline because the tacticity, a measure of the stereoregularity, was only
approximate. But, with the use of the Natta-Zeigler and Phillips catalysts systems PP with greater
stereoregularity was produced giving PP with enhanced physical properties such as increased stiff-
ness, better clarity, and a higher distortion temperature. Today, with better catalysts, including the
soluble metallocene catalysts, the tacticity has been increased so that PP with 99% isotacticity can
be produced. The more traditional Natta-Zeigler catalysts have high catalysts effi ciencies with one
gram of catalysts producing 1kg of PP. This high-catalytic efficiency eliminates the need for catalyst
removal. Most iPP is made using bulk propylene, either as a gas or liquid.
A brief side trip allows some insight into industrial workings. Phillips Petroleum Company was
busily working on ways to improve refinery processes because they were in the 1950s almost solely
a fuel company. Two young chemists, J. Paul Hogan and Robert L. Banks, were trying to develop
catalysts that would act as high-performance gasoline additives when one of the catalyst, mixed
with the petroleum propylene present in a pipe, plugged up the pipe with a whitish, taffy-like mate-
rial. While many companies might have told Hogan and Banks to get back to their original efforts
that were the “bread-and-butter” of the company, instead they were told to investigate the forma-
tion of this off-white material. At this time, most of the known plastics were either too brittle or
softened at too low a temperature for most practical uses. This off-white material, produced from
o
propylene, could be hardened giving a flexible material that melted above 100 C. After some effort,
it was discovered that this catalyst could also be used to give a PE that was superior to the “old” PE.
Eventually, the “old” PE would be given the name of LDPE and the new PE the name of HDPE.
In 1953, a patent was applied for covering both the synthesis of PP and PE under the trade name
of Marlex. Even after a material is discovered many steps are needed before a product becomes
9/14/2010 3:39:10 PM
K10478.indb 172 9/14/2010 3:39:10 PM
K10478.indb 172