Page 214 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 214
Ionic Chain-Reaction and Complex Coordination Polymerization 177
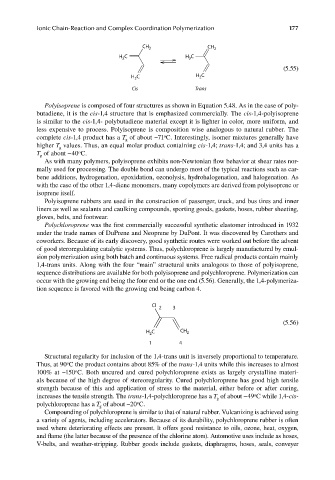
CH
CH 3 2
H C H C
2
3
(5.55)
H C H C
2
2
Cis Trans
Polyisoprene is composed of four structures as shown in Equation 5.48. As in the case of poly-
butadiene, it is the cis-1,4 structure that is emphasized commercially. The cis-1,4-polyisoprene
is similar to the cis-1,4- polybutadiene material except it is lighter in color, more uniform, and
less expensive to process. Polyisoprene is composition wise analogous to natural rubber. The
o
complete cis-1,4 product has a T of about −71 C. Interestingly, isomer mixtures generally have
g
higher T values. Thus, an equal molar product containing cis-1,4; trans-1,4; and 3,4 units has a
g
o
T of about −40 C.
g
As with many polymers, polyisoprene exhibits non-Newtonian flow behavior at shear rates nor-
mally used for processing. The double bond can undergo most of the typical reactions such as car-
bene additions, hydrogenation, epoxidation, ozonolysis, hydrohalogenation, and halogenation. As
with the case of the other 1,4-diene monomers, many copolymers are derived from polyisoprene or
isoprene itself.
Polyisoprene rubbers are used in the construction of passenger, truck, and bus tires and inner
liners as well as sealants and caulking compounds, sporting goods, gaskets, hoses, rubber sheeting,
gloves, belts, and footwear.
Polychloroprene was the first commercially successful synthetic elastomer introduced in 1932
under the trade names of DuPrene and Neoprene by DuPont. It was discovered by Carothers and
coworkers. Because of its early discovery, good synthetic routes were worked out before the advent
of good steroregulating catalytic systems. Thus, polychloroprene is largely manufactured by emul-
sion polymerization using both batch and continuous systems. Free radical products contain mainly
1,4-trans units. Along with the four “main” structural units analogous to those of polyisoprene,
sequence distributions are available for both polyisoprene and polychloroprene. Polymerization can
occur with the growing end being the four end or the one end (5.56). Generally, the 1,4-polymeriza-
tion sequence is favored with the growing end being carbon 4.
Cl 2 3
(5.56)
H C CH 2
2
1 4
Structural regularity for inclusion of the 1,4-trans unit is inversely proportional to temperature.
o
Thus, at 90 C the product contains about 85% of the trans-1,4 units while this increases to almost
100% at −150 C. Both uncured and cured polychloroprene exists as largely crystalline materi-
o
als because of the high degree of stereoregularity. Cured polychloroprene has good high tensile
strength because of this and application of stress to the material, either before or after curing,
increases the tensile strength. The trans-1,4-polychloroprene has a T of about −49 C while 1,4-cis-
o
g
polychloroprene has a T of about −20 C.
o
g
Compounding of polychloroprene is similar to that of natural rubber. Vulcanizing is achieved using
a variety of agents, including accelerators. Because of its durability, polychloroprene rubber is often
used where deteriorating effects are present. It offers good resistance to oils, ozone, heat, oxygen,
and flame (the latter because of the presence of the chlorine atom). Automotive uses include as hoses,
V-belts, and weather-stripping. Rubber goods include gaskets, diaphragms, hoses, seals, conveyer
9/14/2010 3:39:12 PM
K10478.indb 177 9/14/2010 3:39:12 PM
K10478.indb 177