Page 216 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 216
Ionic Chain-Reaction and Complex Coordination Polymerization 179
lubricating oil is not a good solvent for PIB, PIB is present as a coil at room temperature. However, as
the temperature increases, it begins to uncoil acting to counteract the decrease in viscosity of the oil as
the temperature is increased. PIB is also used in sealing applications such as in roof repairs.
It is being used in a number of green chemistry applications. PIB (in the form of PIB succinim-
ide) is added in small amounts to lubricating oils; this reduces the creation of an oil mist, thus lower-
ing the workers, exposure to the oil mist. It is part of Elastol employed to clean up water-borne oil
spills. It increases the crude oil’s viscoelasticity, resulting in an increased ability of the oil to remain
together as it is vacuumed from the water’s surface.
Polyisobutylene acts as a detergent and is used as a fuel additive in diesel fuel reducing fuel injec-
tor fouling, resulting in increased mileage and lowered unwanted emissions. It is also employed as
part of a number of detergent packages that are blended into gasoline and diesel fuels.
5.11 METATHESIS REACTIONS
Chauvin, Grubbs, and Schrock won the 2005 Nobel Prize for developing metathesis reactions. Olefi n
metathesis is a catalytically induced reaction wherein olefins, such as cyclobutene and cyclopentene,
undergo bond reorganization, resulting in the formation of so-called polyalkenamers. Because the
resulting polymers contain double bonds that can be subsequently used to introduce cross-linking,
these materials have been used to produce elastomeric materials as well as plastics. Transition-metal
catalysts are required for these reactions. Catalysts include typical Natta-Ziegler types and other
similar catalysts–cocatalysts combinations. The reactions can be run at room temperature and the
stereoregularity controlled through choice of reaction conditions and catalysts. For instance, the
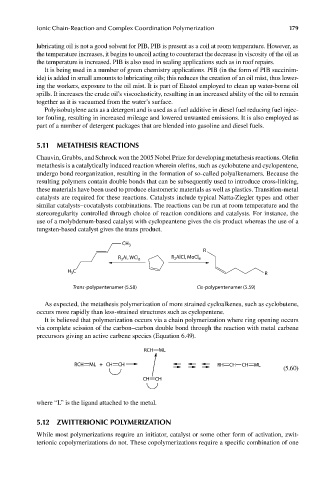
use of a molybdenum-based catalyst with cyclopeantene gives the cis product whereas the use of a
tungsten-based catalyst gives the trans product.
CH 3
R
R Al, WCl 6 R AlCl, MoCl 6
2
3
H C R
3
Trans-polypentenamer (5.58) Cis-polypentenamer (5.59)
As expected, the metathesis polymerization of more strained cycloalkenes, such as cyclobutene,
occurs more rapidly than less-strained structures such as cyclopentene.
It is believed that polymerization occurs via a chain polymerization where ring opening occurs
via complete scission of the carbon–carbon double bond through the reaction with metal carbene
precursors giving an active carbene species (Equation 6.49).
RCH ML
RCH ML + CH CH RH CH CH ML
(5.60)
CH CH
where “L” is the ligand attached to the metal.
5.12 ZWITTERIONIC POLYMERIZATION
While most polymerizations require an initiator, catalyst or some other form of activation, zwit-
terionic copolymerizations do not. These copolymerizations require a specific combination of one
9/14/2010 3:39:12 PM
K10478.indb 179
K10478.indb 179 9/14/2010 3:39:12 PM