Page 24 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 24
Polymer Nomenclature
As with most disciplines, the system followed for naming or defining things is important. Here we
will focus on naming polymers with emphasis on synthetic polymers. Short presentations on how
to name proteins and nucleic acids are provided in Chapter 4 and those for nylons are provided in
Chapter 5.
The fact that synthetic polymer science grew up in many venues before nomenclature groups
were present to assist in standardization of the naming approach resulted in many popular polymers
having several names including common names. Many polymer scientists have not yet accepted the
guidelines given by the offi cial naming committee of the International Union of Pure and Applied
Chemistry, IUPAC, because the common names have gained such widespread acceptance. Although
there is a wide diversity in the practice of naming polymers, we will concentrate on the most utilized
systems.
COMMON NAMES
Little rhyme or reason is associated with many of the common names of polymers. Some names
are derived from the place of origin of the material, such as Hevea brasilliensis— literally “rubber
from Brazil”—for natural rubber. Other polymers were named after their discoverer, as is Bakelite,
the three-dimensional polymer produced by condensation of phenol and formaldehyde, which was
commercialized by Leo Baekeland in 1905.
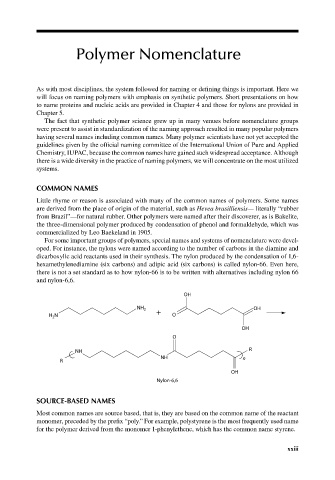
For some important groups of polymers, special names and systems of nomenclature were devel-
oped. For instance, the nylons were named according to the number of carbons in the diamine and
dicarboxylic acid reactants used in their synthesis. The nylon produced by the condensation of 1,6-
hexamethylenediamine (six carbons) and adipic acid (six carbons) is called nylon-66. Even here,
there is not a set standard as to how nylon-66 is to be written with alternatives including nylon 66
and nylon-6,6.
OH
NH 2 + OH
H N O
2
OH
O
NH R
NH n
R
OH
Nylon-6,6
SOURCE-BASED NAMES
Most common names are source based, that is, they are based on the common name of the reactant
monomer, preceded by the prefix “poly.” For example, polystyrene is the most frequently used name
for the polymer derived from the monomer 1-phenylethene, which has the common name styrene.
xxiii
9/14/2010 3:35:44 PM
K10478.indb xxiii
K10478.indb xxiii 9/14/2010 3:35:44 PM