Page 265 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 265
228 Carraher’s Polymer Chemistry
100%
Tie line
Styrene in polymer (%) Free radical, Bz 2 2
Cationic, SnCl
4
O
Anionic, Na
0
0 100%
Styrene in feed (%)
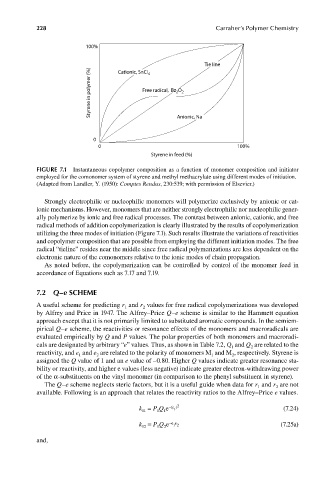
FIGURE 7.1 Instantaneous copolymer composition as a function of monomer composition and initiator
employed for the comonomer system of styrene and methyl methacrylate using different modes of initiation.
(Adapted from Landler, Y. (1950): Comptes Rendus, 230:539; with permission of Elsevier.)
Strongly electrophilic or nucleophilic monomers will polymerize exclusively by anionic or cat-
ionic mechanisms. However, monomers that are neither strongly electrophilic nor nucleophilic gener-
ally polymerize by ionic and free radical processes. The contrast between anionic, cationic, and free
radical methods of addition copolymerization is clearly illustrated by the results of copolymerization
utilizing the three modes of initiation (Figure 7.1). Such results illustrate the variations of reactivities
and copolymer composition that are possible from employing the different initiation modes. The free
radical “tieline” resides near the middle since free radical polymerizations are less dependent on the
electronic nature of the comonomers relative to the ionic modes of chain propagation.
As noted before, the copolymerization can be controlled by control of the monomer feed in
accordance of Equations such as 7.17 and 7.19.
7.2 Q–e SCHEME
A useful scheme for predicting r and r values for free radical copolymerizations was developed
1 2
by Alfrey and Price in 1947. The Alfrey–Price Q–e scheme is similar to the Hammett equation
approach except that it is not primarily limited to substituted aromatic compounds. In the semiem-
pirical Q–e scheme, the reactivities or resonance effects of the monomers and macroradicals are
evaluated empirically by Q and P values. The polar properties of both monomers and macroradi-
cals are designated by arbitrary “e” values. Thus, as shown in Table 7.2, Q and Q are related to the
1 2
reactivity, and e and e are related to the polarity of monomers M and M , respectively. Styrene is
1 2 1 2
assigned the Q value of 1 and an e value of –0.80. Higher Q values indicate greater resonance sta-
bility or reactivity, and higher e values (less negative) indicate greater electron-withdrawing power
of the α-substituents on the vinyl monomer (in comparison to the phenyl substituent in styrene).
The Q–e scheme neglects steric factors, but it is a useful guide when data for r and r are not
1 2
available. Following is an approach that relates the reactivity ratios to the Alfrey–Price e values.
k = P Q e −(e ) 2 (7.24)
1
11 1 1
−e e
k = P Q e 1 2 (7.25a)
12 1 2
and,
9/14/2010 3:39:55 PM
K10478.indb 228 9/14/2010 3:39:55 PM
K10478.indb 228