Page 280 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 280
Copolymerization 243
7.12 EPDM RUBBER
Ethylene–propylene–diene terpolymers rubber’s name is derived from E for ethylene, P for pro-
pylene, D for diene, and M for the ASTM rubber classification. The dienes currently employed are
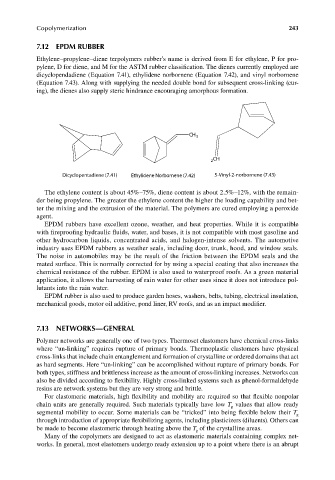
dicyclopendadiene (Equation 7.41), ethylidene norbornene (Equation 7.42), and vinyl norbornene
(Equation 7.43). Along with supplying the needed double bond for subsequent cross-linking (cur-
ing), the dienes also supply steric hindrance encouraging amorphous formation.
CH
3
2 CH
Dicyclopentadiene (7.41) Ethylidene Norbornene (7.42) 5-Vinyl-2-norbornene (7.43)
The ethylene content is about 45%–75%, diene content is about 2.5%–12%, with the remain-
der being propylene. The greater the ethylene content the higher the loading capability and bet-
ter the mixing and the extrusion of the material. The polymers are cured employing a peroxide
agent.
EPDM rubbers have excellent ozone, weather, and heat properties. While it is compatible
with fi reproofing hydraulic fl uids, water, and bases, it is not compatible with most gasoline and
other hydrocarbon liquids, concentrated acids, and halogen-intense solvents. The automotive
industry uses EPDM rubbers as weather seals, including door, trunk, hood, and window seals.
The noise in automobiles may be the result of the friction between the EPDM seals and the
mated surface. This is normally corrected for by using a special coating that also increases the
chemical resistance of the rubber. EPDM is also used to waterproof roofs. As a green material
application, it allows the harvesting of rain water for other uses since it does not introduce pol-
lutants into the rain water.
EPDM rubber is also used to produce garden hoses, washers, belts, tubing, electrical insulation,
mechanical goods, motor oil additive, pond liner, RV roofs, and as an impact modifi er.
7.13 NETWORKS—GENERAL
Polymer networks are generally one of two types. Thermoset elastomers have chemical cross-links
where “un-linking” requires rupture of primary bonds. Thermoplastic elastomers have physical
cross-links that include chain entanglement and formation of crystalline or ordered domains that act
as hard segments. Here “un-linking” can be accomplished without rupture of primary bonds. For
both types, stiffness and brittleness increase as the amount of cross-linking increases. Networks can
also be divided according to flexibility. Highly cross-linked systems such as phenol-formaldehyde
resins are network systems but they are very strong and brittle.
For elastomeric materials, high flexibility and mobility are required so that fl exible nonpolar
chain units are generally required. Such materials typically have low T values that allow ready
g
segmental mobility to occur. Some materials can be “tricked” into being flexible below their T
g
through introduction of appropriate flexibilizing agents, including plasticizers (diluents). Others can
be made to become elastomeric through heating above the T of the crystalline areas.
g
Many of the copolymers are designed to act as elastomeric materials containing complex net-
works. In general, most elastomers undergo ready extension up to a point where there is an abrupt
9/14/2010 3:40:00 PM
K10478.indb 243
K10478.indb 243 9/14/2010 3:40:00 PM