Page 283 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 283
246 Carraher’s Polymer Chemistry
(a) (b)
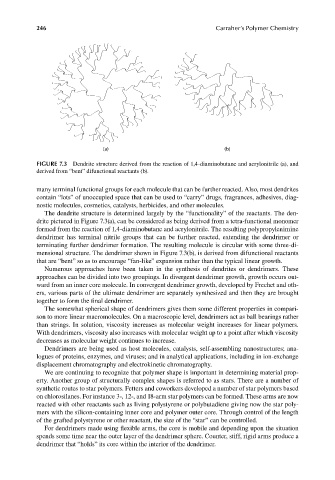
FIGURE 7.3 Dendrite structure derived from the reaction of 1,4-diaminobutane and acrylonitrile (a), and
derived from “bent” difunctional reactants (b).
many terminal functional groups for each molecule that can be further reacted. Also, most dendrites
contain “lots” of unoccupied space that can be used to “carry” drugs, fragrances, adhesives, diag-
nostic molecules, cosmetics, catalysts, herbicides, and other molecules.
The dendrite structure is determined largely by the “functionality” of the reactants. The den-
drite pictured in Figure 7.3(a), can be considered as being derived from a tetra-functional monomer
formed from the reaction of 1,4-diaminobutane and acrylonitrile. The resulting polypropylenimine
dendrimer has terminal nitrile groups that can be further reacted, extending the dendrimer or
terminating further dendrimer formation. The resulting molecule is circular with some three-di-
mensional structure. The dendrimer shown in Figure 7.3(b), is derived from difunctional reactants
that are “bent” so as to encourage “fan-like” expansion rather than the typical linear growth.
Numerous approaches have been taken in the synthesis of dendrites or dendrimers. These
approaches can be divided into two groupings. In divergent dendrimer growth, growth occurs out-
ward from an inner core molecule. In convergent dendrimer growth, developed by Frechet and oth-
ers, various parts of the ultimate dendrimer are separately synthesized and then they are brought
together to form the fi nal dendrimer.
The somewhat spherical shape of dendrimers gives them some different properties in compari-
son to more linear macromolecules. On a macroscopic level, dendrimers act as ball bearings rather
than strings. In solution, viscosity increases as molecular weight increases for linear polymers.
With dendrimers, viscosity also increases with molecular weight up to a point after which viscosity
decreases as molecular weight continues to increase.
Dendrimers are being used as host molecules, catalysts, self-assembling nanostructures; ana-
logues of proteins, enzymes, and viruses; and in analytical applications, including in ion-exchange
displacement chromatography and electrokinetic chromatography.
We are continuing to recognize that polymer shape is important in determining material prop-
erty. Another group of structurally complex shapes is referred to as stars. There are a number of
synthetic routes to star polymers. Fetters and coworkers developed a number of star polymers based
on chlorosilanes. For instance 3-, 12-, and 18-arm star polymers can be formed. These arms are now
reacted with other reactants such as living polystyrene or polybutadiene giving now the star poly-
mers with the silicon-containing inner core and polymer outer core. Through control of the length
of the grafted polystyrene or other reactant, the size of the “star” can be controlled.
For dendrimers made using flexible arms, the core is mobile and depending upon the situation
spends some time near the outer layer of the dendrimer sphere. Counter, stiff, rigid arms produce a
dendrimer that “holds” its core within the interior of the dendrimer.
9/14/2010 3:40:01 PM
K10478.indb 246
K10478.indb 246 9/14/2010 3:40:01 PM