Page 288 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 288
Copolymerization 251
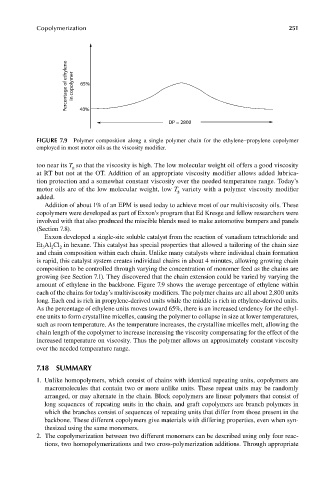
Percentage of ethylene in copolymer 65%
40%
DP = 2800
FIGURE 7.9 Polymer composition along a single polymer chain for the ethylene–propylene copolymer
employed in most motor oils as the viscosity modifi er.
too near its T so that the viscosity is high. The low molecular weight oil offers a good viscosity
g
at RT but not at the OT. Addition of an appropriate viscosity modifier allows added lubrica-
tion protection and a somewhat constant viscosity over the needed temperature range. Today’s
motor oils are of the low molecular weight, low T variety with a polymer viscosity modifi er
g
added.
Addition of about 1% of an EPM is used today to achieve most of our multiviscosity oils. These
copolymers were developed as part of Exxon’s program that Ed Kresge and fellow researchers were
involved with that also produced the miscible blends used to make automotive bumpers and panels
(Section 7.8).
Exxon developed a single-site soluble catalyst from the reaction of vanadium tetrachloride and
Et Al Cl in hexane. This catalyst has special properties that allowed a tailoring of the chain size
2
3
2
and chain composition within each chain. Unlike many catalysts where individual chain formation
is rapid, this catalyst system creates individual chains in about 4 minutes, allowing growing chain
composition to be controlled through varying the concentration of monomer feed as the chains are
growing (see Section 7.1). They discovered that the chain extension could be varied by varying the
amount of ethylene in the backbone. Figure 7.9 shows the average percentage of ethylene within
each of the chains for today’s multiviscosity modifiers. The polymer chains are all about 2,800 units
long. Each end is rich in propylene-derived units while the middle is rich in ethylene-derived units.
As the percentage of ethylene units moves toward 65%, there is an increased tendency for the ethyl-
ene units to form crystalline micelles, causing the polymer to collapse in size at lower temperatures,
such as room temperature. As the temperature increases, the crystalline micelles melt, allowing the
chain length of the copolymer to increase increasing the viscosity compensating for the effect of the
increased temperature on viscosity. Thus the polymer allows an approximately constant viscosity
over the needed temperature range.
7.18 SUMMARY
1. Unlike homopolymers, which consist of chains with identical repeating units, copolymers are
macromolecules that contain two or more unlike units. These repeat units may be randomly
arranged, or may alternate in the chain. Block copolymers are linear polymers that consist of
long sequences of repeating units in the chain, and graft copolymers are branch polymers in
which the branches consist of sequences of repeating units that differ from those present in the
backbone. These different copolymers give materials with differing properties, even when syn-
thesized using the same monomers.
2. The copolymerization between two different monomers can be described using only four reac-
tions, two homopolymerizations and two cross-polymerization additions. Through appropriate
9/14/2010 3:40:06 PM
K10478.indb 251 9/14/2010 3:40:06 PM
K10478.indb 251