Page 286 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 286
Copolymerization 249
chemical processing separations, spent acid regeneration, electrochemical fuel cells, ion-selective separa-
tions, electrodialysis, and in the production of chlorine. It is also employed as a “solid”-state catalyst in
chemical synthesis and processing. Ionomers are also used in blends with other polymers.
7.17 VISCOSITY MODIFIERS
Different mechanisms can be in operation to cause viscosity changes. As noted before, one general
polymer property is that the addition of even small amounts of polymer to a solution can result in
a relatively large increase in viscosity. This increased viscosity is related to the large size of the
polymer chains causing them to be present in several flow planes resulting in what is referred to as
viscous drag. Factors that influence the apparent size of a polymer are reflected in their viscosity
both in solution and bulk.
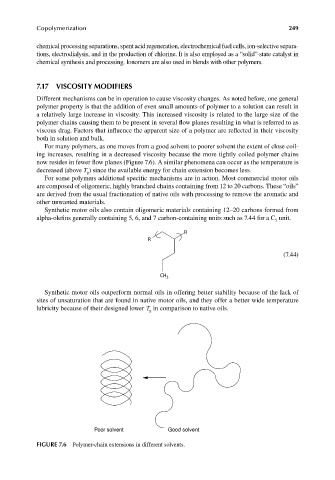
For many polymers, as one moves from a good solvent to poorer solvent the extent of close coil-
ing increases, resulting in a decreased viscosity because the more tightly coiled polymer chains
now resides in fewer flow planes (Figure 7.6). A similar phenomena can occur as the temperature is
decreased (above T ) since the available energy for chain extension becomes less.
g
For some polymers additional specific mechanisms are in action. Most commercial motor oils
are composed of oligomeric, highly branched chains containing from 12 to 20 carbons. These “oils”
are derived from the usual fractionation of native oils with processing to remove the aromatic and
other unwanted materials.
Synthetic motor oils also contain oligomeric materials containing 12–20 carbons formed from
alpha-olefins generally containing 5, 6, and 7 carbon-containing units such as 7.44 for a C unit.
5
R
R
(7.44)
CH 3
Synthetic motor oils outperform normal oils in offering better stability because of the lack of
sites of unsaturation that are found in native motor oils, and they offer a better wide temperature
lubricity because of their designed lower T in comparison to native oils.
g
Poor solvent Good solvent
FIGURE 7.6 Polymer-chain extensions in different solvents.
9/14/2010 3:40:04 PM
K10478.indb 249 9/14/2010 3:40:04 PM
K10478.indb 249