Page 331 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 331
294 Carraher’s Polymer Chemistry
S
S S
S S R
S
S S S S S S S R S
S
S S S S S S
S S S S S S S S
S S S S S S
S S S S
R S
S S S S S S
S S S S S
S
S S S R S
S S R R S
S
S S S S S S S S
S
Slow Fast
S S R
R S S S S S S S S S
S S S S R S S S
S S S S S
S S S S S H 2 O H O S S S
2
S S S S S
S S S S
S S S S S S S S
S S S
S R R S S S S S S S S S
R S S S S S H 2 O S S S S S
S S S S S S S S S H 2 O S H O S
2
S R S
S S
S R S
S
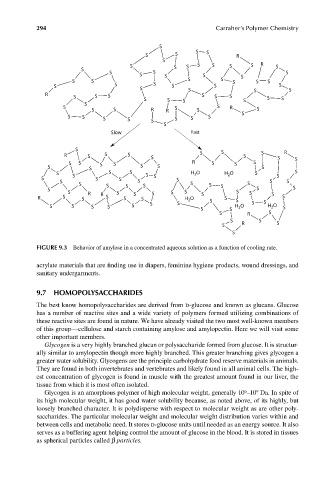
FIGURE 9.3 Behavior of amylose in a concentrated aqueous solution as a function of cooling rate.
acrylate materials that are fi nding use in diapers, feminine hygiene products, wound dressings, and
sanitary undergarments.
9.7 HOMOPOLYSACCHARIDES
The best know homopolysaccharides are derived from d-glucose and known as glucans. Glucose
has a number of reactive sites and a wide variety of polymers formed utilizing combinations of
these reactive sites are found in nature. We have already visited the two most well-known members
of this group—cellulose and starch containing amylose and amylopectin. Here we will visit some
other important members.
Glycogen is a very highly branched glucan or polysaccharide formed from glucose. It is structur-
ally similar to amylopectin though more highly branched. This greater branching gives glycogen a
greater water solubility. Glycogens are the principle carbohydrate food reserve materials in animals.
They are found in both invertebrates and vertebrates and likely found in all animal cells. The high-
est concentration of glycogen is found in muscle with the greatest amount found in our liver, the
tissue from which it is most often isolated.
6
9
Glycogen is an amorphous polymer of high molecular weight, generally 10 –10 Da. In spite of
its high molecular weight, it has good water solubility because, as noted above, of its highly, but
loosely branched character. It is polydisperse with respect to molecular weight as are other poly-
saccharides. The particular molecular weight and molecular weight distribution varies within and
between cells and metabolic need. It stores d-glucose units until needed as an energy source. It also
serves as a buffering agent helping control the amount of glucose in the blood. It is stored in tissues
as spherical particles called β particles.
9/14/2010 3:40:43 PM
K10478.indb 294
K10478.indb 294 9/14/2010 3:40:43 PM