Page 446 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 446
Inorganic Polymers 409
–6
–4
2Si O R Si O + 4O −2 (12.5)
2 7 4 10
(4)
–6
–6
2Si O R Si O + 3O −2 (12.6)
2 7 4 11
(5)
–8
–6
2Si O R Si O + 2O −2 (12.7)
2 7 4 12
(6)
–6
–12
3Si O R Si O + 3O −2 (12.8)
2 7 6 18
(7)
–4
SiO →→→ SiO (12.9)
4 2
(8)
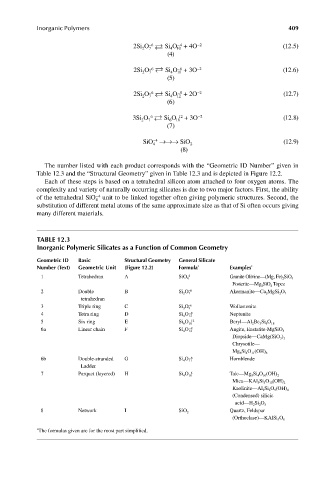
The number listed with each product corresponds with the “Geometric ID Number” given in
Table 12.3 and the “Structural Geometry” given in Table 12.3 and is depicted in Figure 12.2.
Each of these steps is based on a tetrahedral silicon atom attached to four oxygen atoms. The
complexity and variety of naturally occurring silicates is due to two major factors. First, the ability
–4
of the tetrahedral SiO unit to be linked together often giving polymeric structures. Second, the
4
substitution of different metal atoms of the same approximate size as that of Si often occurs giving
many different materials.
TABLE 12.3
Inorganic Polymeric Silicates as a Function of Common Geometry
Geometric ID Basic Structural Geometry General Silicate
Number (Text) Geometric Unit (Figure 12.2) Formula * Examples *
1 Tetrahedran A SiO 4 –4 Granite Olivine—(Mg, Fe) 2 SiO 4
Fosterite—Mg 2 SiO 4 Topez
2 Double B Si 2 O 7 –6 Akermanite—Ca 2 MgSi 2 O 7
tetrahedran
3 Triple ring C Si 3 O 9 –6 Wollastonite
4 Tetra ring D Si 4 O 12 –8 Neptunite
5 Six ring E Si 6 O 18 –12 Beryl—Al 2 Be 3 Si 6 O 18
6a Linear chain F Si 4 O 12 –8 Augite, Enstatite-MgSiO 3
Diopside—CaMg(SiO 3 ) 2
Chrysotile—
Mg 6 Si 4 O 11 (OH) 6
6b Double-stranded G Si 4 O 11 –6 Hornblende
Ladder
7 Parquet (layered) H Si 4 O 10 –4 Talc—Mg 3 Si 4 O 10 (OH) 2
Mica—KAl 3 Si 3 O 10 (OH) 2
Kaolinite—Al 2 Si 2 O 5 (OH) 4
(Condensed) silicic
acid—H 2 Si 2 O 5
8 Network I SiO 2 Quartz, Feldspar
(Orthoclase)—KAlSi 3 O 8
* The formulas given are for the most part simplifi ed.
9/14/2010 3:41:48 PM
K10478.indb 409
K10478.indb 409 9/14/2010 3:41:48 PM