Page 504 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 504
Testing and Spectrometric Characterization of Polymers 467
customary to eliminate this dependence by dividing the charge, Q, by the voltage, V, to get a param-
eter called the capacitance (capacity; C):
C = Q/V (13.5)
and then using the dielectric constant ε, which is defi ned as
ε = C/C (13.6)
o
where C is the capacity of the condenser when the dielectric material is placed between its plates in
a vacuum and C is the empty condenser capacity.
o
Dielectric polarization is the polarized condition in a dielectric resulting from an applied AC or
DC field. The polarizability is the electric dipole moment per unit volume induced by an applied
field or unit effective intensity. The molar polarizability is a measure of the polarizability per molar
volume; thus, it is related to the polarizability of the individual molecules or polymer repeat unit.
Conductivity is a measure of the number of ions per unit volume and their average velocity in the
direction of the applied fi eld. Polarizability is a measure of the number of bound charged particles
per cubic unit and their average displacement in the direction of the applied fi eld.
There are two types of charging currents and condenser charges, which may be described as
rapidly forming or instantaneous polarizations and slowly forming or absorptive polarizations. The
total polarizability of the dielectric is the sum of contributions due to several types of charge dis-
placement in the materials caused by the applied field. The relaxation time is the time required for
polarization to form or disappear. The magnitude of the polarizability, k, of a dielectric is related to
the dielectric constant, ε, as follows:
k = 3(ε −1) / 4π (ε + 2) (13.7)
The terms “polarizability constant” and “dielectric constant” are often used interchangeably in
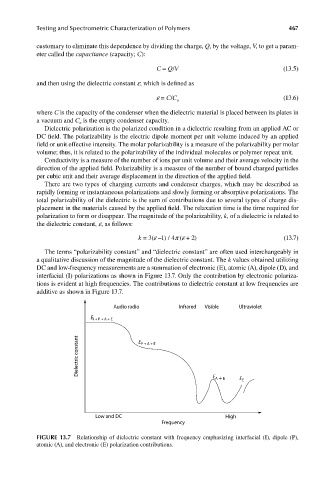
a qualitative discussion of the magnitude of the dielectric constant. The k values obtained utilizing
DC and low-frequency measurements are a summation of electronic (E), atomic (A), dipole (D), and
interfacial (I) polarizations as shown in Figure 13.7. Only the contribution by electronic polariza-
tions is evident at high frequencies. The contributions to dielectric constant at low frequencies are
additive as shown in Figure 13.7.
Audio radio Infrared Visible Ultraviolet
E I + P + A + E
Dielectric constant E P + A + E E A + E E E
Low and DC High
Frequency
FIGURE 13.7 Relationship of dielectric constant with frequency emphasizing interfacial (I), dipole (P),
atomic (A), and electronic (E) polarization contributions.
9/14/2010 3:42:17 PM
K10478.indb 467 9/14/2010 3:42:17 PM
K10478.indb 467