Page 511 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 511
474 Carraher’s Polymer Chemistry
Resistance to biological attack is important for many polymer applications, including almost
all of the biomedical applications, food storage and protection, and coatings where microorganism
destruction is important. Most synthetic polymers are “naturally” resistant to destruction by micro-
organisms. This is particularly true for nonpolar polymers, but less so for condensation polymers
such as nylons and polyesters where microorganisms may recognize similarities to bonds they ordi-
narily hydrolyze. Various preservatives and antimicroorganism additives are added, when appro-
priate, to protect the material against microbial attack. Tests include destructive degradation and
simple growth of the microorganism on the material.
13.12 CHEMICAL RESISTANCE
The classic test for chemical resistance (ASTM D-543) measures the percentage weight change of
test samples after immersion in different liquid systems. Tests for chemical resistance have been
extended to include changes in mechanical properties after immersion. Since chemical attack
involves changes in chemical structure, it can be readily observed by many instrumental methods
that measure chemical structure, in particular, surface structure.
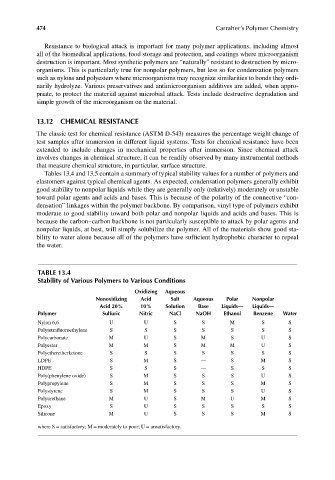
Tables 13.4 and 13.5 contain a summary of typical stability values for a number of polymers and
elastomers against typical chemical agents. As expected, condensation polymers generally exhibit
good stability to nonpolar liquids while they are generally only (relatively) moderately or unstable
toward polar agents and acids and bases. This is because of the polarity of the connective “con-
densation” linkages within the polymer backbone. By comparison, vinyl type of polymers exhibit
moderate to good stability toward both polar and nonpolar liquids and acids and bases. This is
because the carbon–carbon backbone is not particularly susceptible to attack by polar agents and
nonpolar liquids, at best, will simply solubilize the polymer. All of the materials show good sta-
bility to water alone because all of the polymers have sufficient hydrophobic character to repeal
the water.
TABLE 13.4
Stability of Various Polymers to Various Conditions
Oxidizing Aqueous
Nonoxidizing Acid Salt Aqueous Polar Nonpolar
Acid 20% 10% Solution Base Liquids— Liquids—
Polymer Sulfuric Nitric NaCl NaOH Ethanol Benzene Water
Nylon 6,6 U U S S M S S
Polytetrafl uoroethylene S S S S S S S
Polycarbonate M U S M S U S
Polyester M M S M M U S
Polyetheretherketone S S S S S S S
LDPE S M S — S M S
HDPE S S S — S S S
Poly(phenylene oxide) S M S S S U S
Polypropylene S M S S S M S
Polystyrene S M S S S U S
Polyurethane M U S M U M S
Epoxy S U S S S S S
Silicone M U S S S M S
where S = satisfactory; M = moderately to poor; U = unsatisfactory.
9/14/2010 3:42:19 PM
K10478.indb 474 9/14/2010 3:42:19 PM
K10478.indb 474