Page 514 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 514
Testing and Spectrometric Characterization of Polymers 477
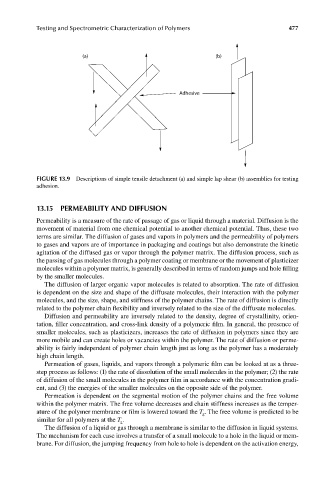
(a) (b)
Adhesive
FIGURE 13.9 Descriptions of simple tensile detachment (a) and simple lap shear (b) assemblies for testing
adhesion.
13.15 PERMEABILITY AND DIFFUSION
Permeability is a measure of the rate of passage of gas or liquid through a material. Diffusion is the
movement of material from one chemical potential to another chemical potential. Thus, these two
terms are similar. The diffusion of gases and vapors in polymers and the permeability of polymers
to gases and vapors are of importance in packaging and coatings but also demonstrate the kinetic
agitation of the diffused gas or vapor through the polymer matrix. The diffusion process, such as
the passing of gas molecules through a polymer coating or membrane or the movement of plasticizer
molecules within a polymer matrix, is generally described in terms of random jumps and hole fi lling
by the smaller molecules.
The diffusion of larger organic vapor molecules is related to absorption. The rate of diffusion
is dependent on the size and shape of the diffusate molecules, their interaction with the polymer
molecules, and the size, shape, and stiffness of the polymer chains. The rate of diffusion is directly
related to the polymer chain flexibility and inversely related to the size of the diffusate molecules.
Diffusion and permeability are inversely related to the density, degree of crystallinity, orien-
tation, filler concentration, and cross-link density of a polymeric film. In general, the presence of
smaller molecules, such as plasticizers, increases the rate of diffusion in polymers since they are
more mobile and can create holes or vacancies within the polymer. The rate of diffusion or perme-
ability is fairly independent of polymer chain length just as long as the polymer has a moderately
high chain length.
Permeation of gases, liquids, and vapors through a polymeric film can be looked at as a three-
step process as follows: (1) the rate of dissolution of the small molecules in the polymer; (2) the rate
of diffusion of the small molecules in the polymer film in accordance with the concentration gradi-
ent, and (3) the energies of the smaller molecules on the opposite side of the polymer.
Permeation is dependent on the segmental motion of the polymer chains and the free volume
within the polymer matrix. The free volume decreases and chain stiffness increases as the temper-
ature of the polymer membrane or film is lowered toward the T . The free volume is predicted to be
g
similar for all polymers at the T .
g
The diffusion of a liquid or gas through a membrane is similar to the diffusion in liquid systems.
The mechanism for each case involves a transfer of a small molecule to a hole in the liquid or mem-
brane. For diffusion, the jumping frequency from hole to hole is dependent on the activation energy,
9/14/2010 3:42:19 PM
K10478.indb 477 9/14/2010 3:42:19 PM
K10478.indb 477