Page 592 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 592
Synthesis of Reactants and Intermediates for Polymers 555
Hexamethylene tetramine
N
N N
N
NH 3
O
O H H
Cl Cl
Phosgene O 2
H C CH 2
H C OH 2
3
Ethylene
CO, H 2 O
CH 4 CO + H 2 H 3 C OH OH
H H HO
Ethylene glycol
O
H C H 2 C CH 3
3
CO 2 O H O
NH 3
NH 3 O 2, CH 4 O
H 2 N NH 2 Vinyl acetate
HC N
O O
OH H 3 C
H C OH H C CH 3
2
3
O
H C
3
C O CH
H 3
NH 2 N 3
O CH 3 Methyl methacrylate
N N
N N NH
H 2 2
Melamine
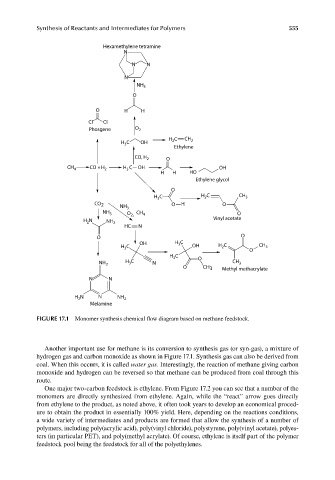
FIGURE 17.1 Monomer synthesis chemical flow diagram based on methane feedstock.
Another important use for methane is its conversion to synthesis gas (or syn-gas), a mixture of
hydrogen gas and carbon monoxide as shown in Figure 17.1. Synthesis gas can also be derived from
coal. When this occurs, it is called water gas. Interestingly, the reaction of methane giving carbon
monoxide and hydrogen can be reversed so that methane can be produced from coal through this
route.
One major two-carbon feedstock is ethylene. From Figure 17.2 you can see that a number of the
monomers are directly synthesized from ethylene. Again, while the “react” arrow goes directly
from ethylene to the product, as noted above, it often took years to develop an economical proced-
ure to obtain the product in essentially 100% yield. Here, depending on the reactions conditions,
a wide variety of intermediates and products are formed that allow the synthesis of a number of
polymers, including poly(acrylic acid), poly(vinyl chloride), polystyrene, poly(vinyl acetate), polyes-
ters (in particular PET), and poly(methyl acrylate). Of course, ethylene is itself part of the polymer
feedstock pool being the feedstock for all of the polyethylenes.
9/14/2010 3:43:17 PM
K10478.indb 555
K10478.indb 555 9/14/2010 3:43:17 PM