Page 593 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 593
556 Carraher’s Polymer Chemistry
Vinyl chloride
H C
2
CH 2
Acrylic acid Cl
O
CH 3
OH
CH 2 Cl
Cl Styrene
+
O
H C CH 2
2
C
H 3 O
OH
CH 3
O O
Vinyl acetate
O HC N CH 2 OH H 2 C CH 3
O
CH 2 Acrylic acid
O
N
N Methyl acrylate
PEG OH HO
HO
H C
2
Ethylene glycol Acrylonitrile
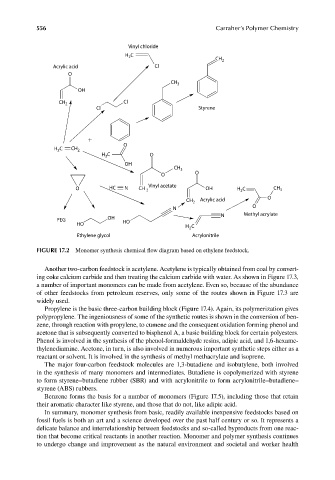
FIGURE 17.2 Monomer synthesis chemical flow diagram based on ethylene feedstock.
Another two-carbon feedstock is acetylene. Acetylene is typically obtained from coal by convert-
ing coke calcium carbide and then treating the calcium carbide with water. As shown in Figure 17.3,
a number of important monomers can be made from acetylene. Even so, because of the abundance
of other feedstocks from petroleum reserves, only some of the routes shown in Figure 17.3 are
widely used.
Propylene is the basic three-carbon building block (Figure 17.4). Again, its polymerization gives
polypropylene. The ingeniousness of some of the synthetic routes is shown in the conversion of ben-
zene, through reaction with propylene, to cumene and the consequent oxidation forming phenol and
acetone that is subsequently converted to bisphenol A, a basic building block for certain polyesters.
Phenol is involved in the synthesis of the phenol-formaldehyde resins, adipic acid, and 1,6-hexame-
thylenediamine. Acetone, in turn, is also involved in numerous important synthetic steps either as a
reactant or solvent. It is involved in the synthesis of methyl methacrylate and isoprene.
The major four-carbon feedstock molecules are 1,3-butadiene and isobutylene, both involved
in the synthesis of many monomers and intermediates. Butadiene is copolymerized with styrene
to form styrene–butadiene rubber (SBR) and with acrylonitrile to form acrylonitrile–butadiene–
styrene (ABS) rubbers.
Benzene forms the basis for a number of monomers (Figure 17.5), including those that retain
their aromatic character like styrene, and those that do not, like adipic acid.
In summary, monomer synthesis from basic, readily available inexpensive feedstocks based on
fossil fuels is both an art and a science developed over the past half century or so. It represents a
delicate balance and interrelationship between feedstocks and so-called byproducts from one reac-
tion that become critical reactants in another reaction. Monomer and polymer synthesis continues
to undergo change and improvement as the natural environment and societal and worker health
9/14/2010 3:43:18 PM
K10478.indb 556 9/14/2010 3:43:18 PM
K10478.indb 556