Page 663 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 663
626 Carraher’s Polymer Chemistry
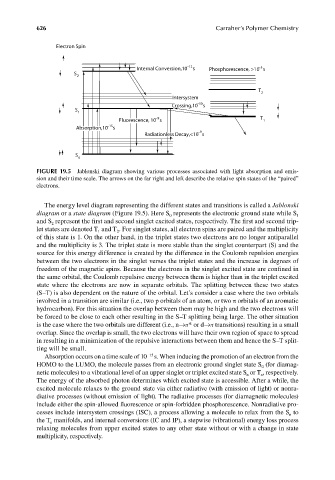
Electron Spin
–3
Internal Conversion,10 –12 s Phosphorescence, >10 s
S
2
T 2
Intersystem
–10
Crossing,10 s
S
1
Fluorescence, 10 –9 s T 1
Absorption,10 –15 s
–9
Radiationless Decay,<10 s
S
o
FIGURE 19.5 Jablonski diagram showing various processes associated with light absorption and emis-
sion and their time scale. The arrows on the far right and left describe the relative spin states of the “paired”
electrons.
The energy level diagram representing the different states and transitions is called a Jablonski
diagram or a state diagram (Figure 19.5). Here S represents the electronic ground state while S
0
1
and S represent the first and second singlet excited states, respectively. The first and second trip-
2
let states are denoted T and T . For singlet states, all electron spins are paired and the multiplicity
2
1
of this state is 1. On the other hand, in the triplet states two electrons are no longer antiparallel
and the multiplicity is 3. The triplet state is more stable than the singlet counterpart (S) and the
source for this energy difference is created by the difference in the Coulomb repulsion energies
between the two electrons in the singlet verses the triplet states and the increase in degrees of
freedom of the magnetic spins. Because the electrons in the singlet excited state are confi ned in
the same orbital, the Coulomb repulsive energy between them is higher than in the triplet excited
state where the electrons are now in separate orbitals. The splitting between these two states
(S–T) is also dependent on the nature of the orbital. Let’s consider a case where the two orbitals
involved in a transition are similar (i.e., two p orbitals of an atom, or two π orbitals of an aromatic
hydrocarbon). For this situation the overlap between them may be high and the two electrons will
be forced to be close to each other resulting in the S–T splitting being large. The other situation
is the case where the two orbitals are different (i.e., n→π* or d→π transitions) resulting in a small
overlap. Since the overlap is small, the two electrons will have their own region of space to spread
in resulting in a minimization of the repulsive interactions between them and hence the S–T split-
ting will be small.
Absorption occurs on a time scale of 10 −15 s. When inducing the promotion of an electron from the
HOMO to the LUMO, the molecule passes from an electronic ground singlet state S (for diamag-
0
netic molecules) to a vibrational level of an upper singlet or triplet excited state S or T , respectively.
n
n
The energy of the absorbed photon determines which excited state is accessible. After a while, the
excited molecule relaxes to the ground state via either radiative (with emission of light) or nonra-
diative processes (without emission of light). The radiative processes (for diamagnetic molecules)
include either the spin-allowed fl uorescence or spin-forbidden phosphorescence. Nonradiative pro-
cesses include intersystem crossings (ISC), a process allowing a molecule to relax from the S to
n
the T manifolds, and internal conversions (IC and IP), a stepwise (vibrational) energy loss process
n
relaxing molecules from upper excited states to any other state without or with a change in state
multiplicity, respectively.
K10478.indb 626 9/14/2010 3:43:59 PM
9/14/2010 3:43:59 PM
K10478.indb 626