Page 672 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 672
Selected Topics 635
These two are used either separately or as a mixture. Because of the presence of the somewhat
flexible ethylene oxide and related units and use of appropriate fillers, these materials give compos-
ite fi llings with lower polymerization shrinkage, enhanced mechanical properties, lower solubility
and water adsorption, better thermal expansion characteristics, good biocompatibility, with aes-
thetic properties closely matching those of the tooth itself.
Some other fillings employ urethanedimethacrylate (1,6-bis(methacryloxy-2-ethoxycarbonylamino)-
2,4,4-trimethylhexane) (UDMA) in place of bis-GMA. This is an active area of research with new
monomer systems being introduced in an ongoing manner.
Cavity varnishes are used to seal the exposed dentinal tubules and protect the pulp from the irri-
tation of chemicals in the filling materials. They are generally largely natural rubber or a synthetic
polymeric resin such as 2-hydroxyethyl methacrylate (HEMA).
Fillings should be viewed as nonpermanent with a lifetime often of a decade. Many materials are
employed in fillings, including metals, alloys, amalgams, composites, and glass ionomer cements.
Glass ionomer cements are a mixture of glass and an ionomer referred to as a polyalkonic acid,
which is simply an ionomer with carboxylic salt groups as the chelating moieties. The ionometric
polymer chelates both the tooth and glass binding them together into the filling. Today’s glass iono-
mer cements are especially designed for dental application. For instance, many contain releasable
fluoride that prevents carious lesions with the fluoride content recharged through use of fl uoride-
containing toothpaste. Newer formulations containing light-cured resins do not release fl uoride as
readily.
Almost all denture bases are made of methacrylic (acrylic) resins that give good fit and a natural
appearance. A compression molding process is used where the monomer-polymer dough or slurry
contain poly(methyl methacrylate) or poly(methyl acrylate). Often there is a change in the contour
of the soft tissue and a liner is fitted onto the denture base. Silicon reliners are often used for this
purpose.
Plastic acrylic denture teeth are made by injection or transfer molding. Acrylic teeth have a
higher strength than porcelain teeth and break less readily. However, they cold flow, have a greater
water absorption, and they have a higher wear rate than porcelain teeth.
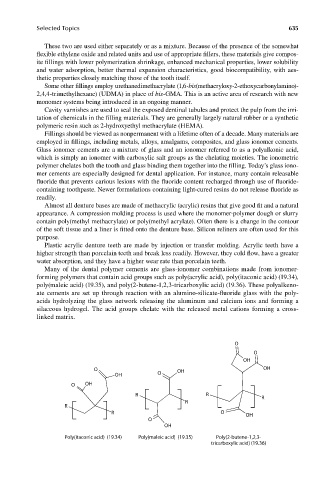
Many of the dental polymer cements are glass-ionomer combinations made from ionomer-
forming polymers that contain acid groups such as poly(acrylic acid), poly(itaconic acid) (19.34),
poly(maleic acid) (19.35), and poly(2-butene-1,2,3-tricarboxylic acid) (19.36). These polyalkeno-
ate cements are set up through reaction with an alumino-silicate-fluoride glass with the poly-
acids hydrolyzing the glass network releasing the aluminum and calcium ions and forming a
silaceous hydrogel. The acid groups chelate with the released metal cations forming a cross-
linked matrix.
O
O
OH
O OH OH
OH O
O OH
R R
R
R
R
R O
OH
O
OH
Poly(itaconic acid) (19.34) Poly(maleic acid) (19.35) Poly(2-butene-1,2,3-
tricarboxylic acid) (19.36)
9/14/2010 3:44:02 PM
K10478.indb 635
K10478.indb 635 9/14/2010 3:44:02 PM