Page 84 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 84
Polymer Structure (Morphology) 47
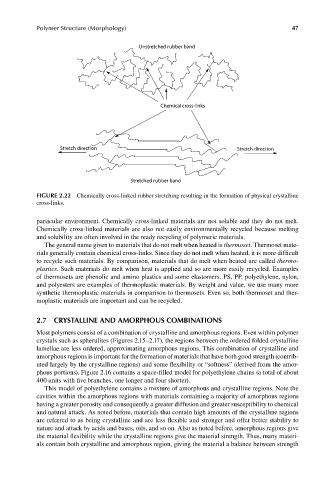
Unstretched rubber band
Chemical cross-links
Stretch direction Stretch direction
Stretched rubber band
FIGURE 2.22 Chemically cross-linked rubber stretching resulting in the formation of physical crystalline
cross-links.
particular environment. Chemically cross-linked materials are not soluble and they do not melt.
Chemically cross-linked materials are also not easily environmentally recycled because melting
and solubility are often involved in the ready recycling of polymeric materials.
The general name given to materials that do not melt when heated is thermoset. Thermoset mate-
rials generally contain chemical cross-links. Since they do not melt when heated, it is more diffi cult
to recycle such materials. By comparison, materials that do melt when heated are called thermo-
plastics. Such materials do melt when heat is applied and so are more easily recycled. Examples
of thermosets are phenolic and amino plastics and some elastomers. PS, PP, polyethylene, nylon,
and polyesters are examples of thermoplastic materials. By weight and value, we use many more
synthetic thermoplastic materials in comparison to thermosets. Even so, both thermoset and ther-
moplastic materials are important and can be recycled.
2.7 CRYSTALLINE AND AMORPHOUS COMBINATIONS
Most polymers consist of a combination of crystalline and amorphous regions. Even within polymer
crystals such as spherulites (Figures 2.15–2.17), the regions between the ordered folded crystalline
lamellae are less ordered, approximating amorphous regions. This combination of crystalline and
amorphous regions is important for the formation of materials that have both good strength (contrib-
uted largely by the crystalline regions) and some flexibility or “softness” (derived from the amor-
phous portions). Figure 2.16 contains a space-filled model for polyethylene chains (a total of about
400 units with five branches, one longer and four shorter).
This model of polyethylene contains a mixture of amorphous and crystalline regions. Note the
cavities within the amorphous regions with materials containing a majority of amorphous regions
having a greater porosity and consequently a greater diffusion and greater susceptibility to chemical
and natural attack. As noted before, materials that contain high amounts of the crystalline regions
are referred to as being crystalline and are less flexible and stronger and offer better stability to
nature and attack by acids and bases, oils, and so on. Also as noted before, amorphous regions give
the material flexibility while the crystalline regions give the material strength. Thus, many materi-
als contain both crystalline and amorphous region, giving the material a balance between strength
9/14/2010 3:36:12 PM
K10478.indb 47 9/14/2010 3:36:12 PM
K10478.indb 47