Page 193 - Cascade biocatalysis
P. 193
7.3 N-Carbamoyl-β-Alanine Amidohydrolase 169
different to the natural ones. The capacity of one enzyme to metabolize structurally
distinct substrates or to convert a single substrate into multiple products has been
termed promiscuity, and it has stimulated research interest due to the concept’s
many possible implications [55]. Promiscuity can be broken down into conditions
promiscuity, catalytic promiscuity, and substrate promiscuity. The latter refers to
enzymes with relaxed or broad substrate specificity, and from the industrial point
of view, it is very interesting to have enzymes with high activity against different
suitable and cheap substrates. The quantification of substrate promiscuity p can
i
be calculated from catalytic efficiency (e) as shown in Eq. (7.1), where k is the rate
cat
at which product is generated by an enzyme under saturating substrate concentra-
tions. K is the Michaelis constant and represents the concentration of substrate
m
that yields a half-maximal rate.
= cat (7.1)
m
After calculating the catalytic efficiency e for different substrates N, the value p
i i
is obtained by Eq. (7.2). This indicates the probability that the ith substrate will be
the first to metabolize when an enzyme is simultaneously exposed to equal, low
concentrations of all N substrates.
= (7.2)
∑
=1
Atβcar enzyme has shown its capacity to hydrolyze N-carbamoyl compounds
to α-, β-, γ-, and δ-amino acids, making it an attractive tool for the production
of several interesting compounds (Table 7.1). This evaluation confirms it as a
ureidohydrolase and mainly a β-ureidopropionase or NCβAA [48]. Atβcar is mod-
estly promiscuous with a 55% probability ‘‘p ’’ of using N-carbamoyl-l-methionine
i
as substrate, followed by N-carbamoyl-l-alanine (17%) and N-carbamoyl-β-alanine
(8%), the first β precursor. These findings confirm this enzyme to be the sec-
ond β-ureidopropionase with broad activity toward N-carbamoyl-α-amino acids
[32, 49, 50], and corroborate the relationships between β-ureidopropionases and
l-carbamoylases. Atβcar substrate promiscuity was also studied in terms of its
ability to produce the same α-or β-amino acid from different precursors. Thus,
for methionine production, the best affinity constant was also obtained when the
←−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
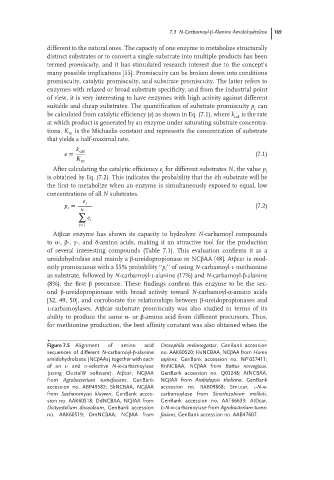
Figure 7.5 Alignment of amino acid Drosophila melanogaster, GenBank accession
sequences of different N-carbamoyl-β-alanine no. AAK60520; HsNCBAA, NCβAA from Homo
amidohydrolases (NCβAAs) together with each sapiens, GenBank accession no. NP˙057411;
of an L-and D-selective N-α-carbamoylase RnNCBAA, NCβAA from Rattus norvegicus,
(using ClustalW software). Atβcar, NCβAA GenBank accession no. Q03248; AtNCBAA,
from Agrobacterium tumefaciens, GenBank NCβAA from Arabidopsis thaliana, GenBank
accession no. ABP49582; SkNCBAA, NCβAA accession no. BAB09868; SmLcar, L-N-α-
from Sacharomyces kluyveri, GenBank acces- carbamoylase from Sinorhizobium meliloti,
sion no. AAK60518; DdNCBAA, NCβAA from GenBank accession no. AAT66633; AtDcar,
Dictyostelium discoideum, GenBank accession D-N-α-carbamoylase from Agrobacterium tume-
no. AAK60519; DmNCBAA, NCβAA from faciens, GenBank accession no. AAB47607.