Page 404 - Cascade biocatalysis
P. 404
380 17 Enzymatic Generation of Sialoconjugate Diversity
high costs and consequently extended reaction times from use of lower quantities
of a less stable enzyme.
OH O CMP HOOC
3 CSS 2,6SiaT 3 OH OH
R 3 R O O
1 O CO H 1 R O CO H 1 O
R 2 R 2 R HO O O
OH OH OH HO O
2 CTP PP 2 2 OH
R i R CMP R OH Acr
PPase AP
2 P i OH OH Cytidine + P
HO O i
HO O O O
OH HO
36 OH Acr
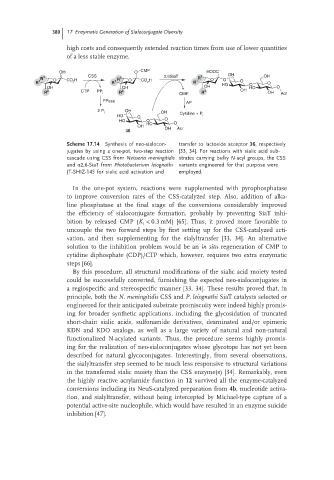
Scheme 17.14 Synthesis of neo-sialocon- transfer to lactoside acceptor 36, respectively
jugates by using a one-pot, two-step reaction [33, 34]. For reactions with sialic acid sub-
cascade using CSS from Neisseria meningitidis strates carrying bulky N-acyl groups, the CSS
and α2,6-SiaT from Photobacterium leiognathi variants engineered for that purpose were
JT-SHIZ-145 for sialic acid activation and employed.
In the one-pot system, reactions were supplemented with pyrophosphatase
to improve conversion rates of the CSS-catalyzed step. Also, addition of alka-
line phosphatase at the final stage of the conversions considerably improved
the efficiency of sialoconjugate formation, probably by preventing SiaT inhi-
bition by released CMP (K < 0.3 mM) [65]. Thus, it proved more favorable to
i
uncouple the two forward steps by first setting up for the CSS-catalyzed acti-
vation, and then supplementing for the sialyltransfer [33, 34]. An alternative
solution to the inhibition problem would be an in situ regeneration of CMP to
cytidine diphosphate (CDP)/CTP which, however, requires two extra enzymatic
steps [66].
By this procedure, all structural modifications of the sialic acid moiety tested
could be successfully converted, furnishing the expected neo-sialoconjugates in
a regiospecific and stereospecific manner [33, 34]. These results proved that, in
principle, both the N. meningitidis CSS and P. leiognathi SiaT catalysts selected or
engineered for their anticipated substrate promiscuity were indeed highly promis-
ing for broader synthetic applications, including the glycosidation of truncated
short-chain sialic acids, sulfonamide derivatives, deaminated and/or epimeric
KDN and KDO analogs, as well as a large variety of natural and non-natural
functionalized N-acylated variants. Thus, the procedure seems highly promis-
ing for the realization of neo-sialoconjugates whose glycotope has not yet been
described for natural glycoconjugates. Interestingly, from several observations,
the sialyltransfer step seemed to be much less responsive to structural variations
in the transferred sialic moiety than the CSS enzyme(s) [34]. Remarkably, even
the highly reactive acrylamide function in 12 survived all the enzyme-catalyzed
conversions including its NeuS-catalyzed preparation from 4b, nucleotide activa-
tion, and sialyltransfer, without being intercepted by Michael-type capture of a
potential active-site nucleophile, which would have resulted in an enzyme suicide
inhibition [47].