Page 405 - Cascade biocatalysis
P. 405
17.3 Cascade Synthesis of neo-Sialoconjugates 381
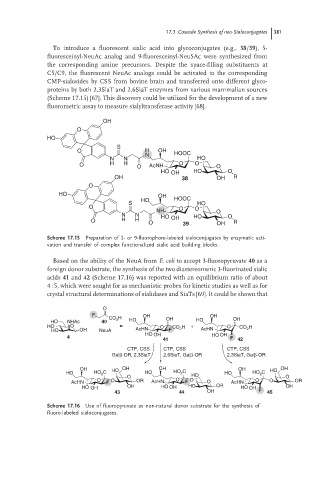
To introduce a fluorescent sialic acid into glycoconjugates (e.g., 38/39), 5-
fluoresceinyl-NeuAc analog and 9-fluoresceinyl-Neu5Ac were synthesized from
the corresponding amine precursors. Despite the space-filling substituents at
C5/C9, the fluorescent NeuAc analogs could be activated to the corresponding
CMP-sialosides by CSS from bovine brain and transferred onto different glyco-
proteins by both 2,3SiaT and 2,6SiaT enzymes from various mammalian sources
(Scheme 17.15) [67]. This discovery could be utilized for the development of a new
fluorometric assay to measure sialyltransferase activity [68].
OH
O
HO
S
O H OH
N HOOC
N N HO
O H H O AcNH O O O
HO OH HO O
OH 38 OH R
O
HO OH
HO HOOC
S HO
O O O
NH O
N N HO HO O
O H H O OH 39 OH R
Scheme 17.15 Preparation of 5- or 9-fluorophore-labeled sialoconjugates by enzymatic acti-
vation and transfer of complex functionalized sialic acid building blocks.
Based on the ability of the NeuA from E. coli to accept 3-fluoropyruvate 40 as a
foreign donor substrate, the synthesis of the two diastereomeric 3-fluorinated sialic
acids 41 and 42 (Scheme 17.16) was reported with an equilibrium ratio of about
4 : 5, which were sought for as mechanistic probes for kinetic studies as well as for
crystal structural determinations of sialidases and SiaTs [69]. It could be shown that
O
F OH OH
CO H OH OH
HO NHAc 40 2 HO HO
HO O O CO H + O CO H
HO OH NeuA AcHN F 2 AcHN 2
HO OH OH
4 HO F
41 42
CTP, CSS CTP, CSS CTP, CSS
Galβ-OR, 2,3SiaT 2,6SiaT, Galβ-OR 2,3SiaT, Galβ-OR
OH HO OH OH OH HO OH
HO HO C HO HO 2 C HO HO 2 C
2
O HO O
AcHN O F O OR AcHN O F O O AcHN O O OR
HO OH OH HO OH HO OR HO OH OH
43 44 OH F 45
Scheme 17.16 Use of fluoropyruvate as non-natural donor substrate for the synthesis of
fluoro-labeled sialoconjugates.