Page 102 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 102
88 hydrolysis, oxidation and reduction
6.1 ASYMMETRIC EPOXIDATION OF DISUBSTITUTED Z-ALKENES
USING A CHIRAL SALEN±MANGANESE COMPLEX [1]
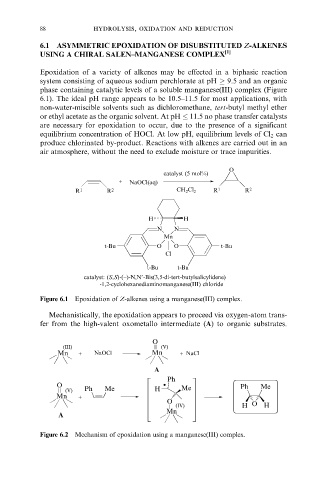
Epoxidation of a variety of alkenes may be effected in a biphasic reaction
system consisting of aqueous sodium perchlorate at pH 9.5 and an organic
phase containing catalytic levels of a soluble manganese(III) complex (Figure
6.1). The ideal pH range appears to be 10.5±11.5 for most applications, with
non-water-miscible solvents such as dichloromethane, tert-butyl methyl ether
or ethyl acetate as the organic solvent. At pH 11.5 no phase transfer catalysts
are necessary for epoxidation to occur, due to the presence of a significant
equilibrium concentration of HOCl. At low pH, equilibrium levels of Cl 2 can
produce chlorinated by-product. Reactions with alkenes are carried out in an
air atmosphere, without the need to exclude moisture or trace impurities.
O
catalyst (5 mol%)
+ NaOCl(aq)
R 1 R 2 CH 2 Cl 2 R 1 R 2
H H
N N
Mn
t-Bu O O t-Bu
Cl
t-Bu t-Bu
catalyst: (S,S)-(−)-N,N9-Bis(3,5-di-tert-butylsalicylidene)
-1,2-cyclohexanediaminomanganese(III) chloride
Figure 6.1 Epoxidation of Z-alkenes using a manganese(III) complex.
Mechanistically, the epoxidation appears to proceed via oxygen-atom trans-
fer from the high-valent oxometallo intermediate (A) to organic substrates.
O
(III) (V)
Mn + NaOCl Mn + NaCl
A
Ph
O Me Ph Me
(V) Ph Me H
Mn +
O
(IV) H O H
Mn
A
Figure 6.2 Mechanism of epoxidation using a manganese(III) complex.