Page 98 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 98
84 hydrolysis, oxidation and reduction
1
. ee 88 % determined by H NMR analysis of the Mosher ester; [a] D 25
ÿ22(c 1:33, CHCl 3 ).
. 1 H NMR (200 MHz, CDCl 3 ): d 3.88 (dd, J2, 13 Hz, 1H), 3.59 (ddd, J5, 7,
10 Hz, 1H), 2.87±2.96 (m, 2H), 2.68 (br s), 1.24±1.55 (m, 14H), 0.85 (t, J
7 Hz, 3H).
ÿ1
. FTIR (CHCl 3 cm ): 1237 (in phase epoxy); 933±817 (out of phase epoxy).
Utility and Scope
Use of poly(octamethylene tartrate) in place of dialkyl tartrates offers practical
utility since the branched polymers yield hetereogeneous Ti complex catalysts
which can be removed by filtration. Overall the work-up procedure is con-
siderably simplified relative to the conventional Sharpless system. In addition,
significant induction is shown in the epoxidation of (Z)-allylic alcohols [7] and
even with homoallylic [8] species where the dialkyltartrates give very poor results
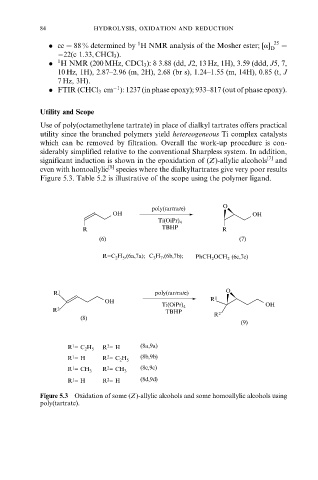
Figure 5.3. Table 5.2 is illustrative of the scope using the polymer ligand.
O
poly(tartrate)
OH OH
Ti(OiPr) 4
R TBHP R
(6) (7)
R=C 2 H 5 ,(6a,7a); C 3 H 7 ,(6b,7b); PhCH 2 OCH 2 (6c,7c)
R 1 poly(tartrate) O
OH R 1
Ti(OiPr) 4 OH
R 2 TBHP
(8) R 2
(9)
R 2 = H (8a,9a)
R 1 = C 2 H 5
R 1 = H R 2 = C 2 H 5 (8b,9b)
(8c,9c)
R 1 = CH 3 R 2 = CH 3
R 1 = H R 2 = H (8d,9d)
Figure 5.3 Oxidation of some (Z)-allylic alcohols and some homoallylic alcohols using
poly(tartrate).