Page 25 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 25
GENERAL OR UNIFORM OR QUASI-UNIFORM CORROSION 3
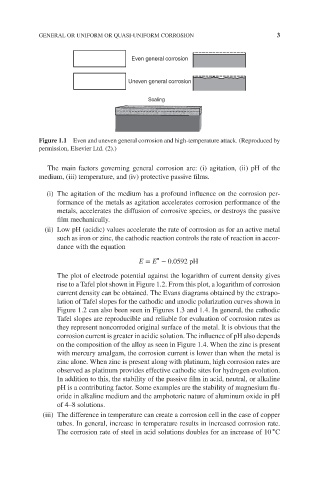
Even general corrosion
Uneven general corrosion
Scaling
Figure 1.1 Even and uneven general corrosion and high-temperature attack. (Reproduced by
permission, Elsevier Ltd. (2).)
The main factors governing general corrosion are: (i) agitation, (ii) pH of the
medium, (iii) temperature, and (iv) protective passive films.
(i) The agitation of the medium has a profound influence on the corrosion per-
formance of the metals as agitation accelerates corrosion performance of the
metals, accelerates the diffusion of corrosive species, or destroys the passive
film mechanically.
(ii) Low pH (acidic) values accelerate the rate of corrosion as for an active metal
such as iron or zinc, the cathodic reaction controls the rate of reaction in accor-
dance with the equation
∘
E = E − 0.0592 pH
The plot of electrode potential against the logarithm of current density gives
rise to a Tafel plot shown in Figure 1.2. From this plot, a logarithm of corrosion
current density can be obtained. The Evans diagrams obtained by the extrapo-
lation of Tafel slopes for the cathodic and anodic polarization curves shown in
Figure 1.2 can also been seen in Figures 1.3 and 1.4. In general, the cathodic
Tafel slopes are reproducible and reliable for evaluation of corrosion rates as
they represent noncorroded original surface of the metal. It is obvious that the
corrosion current is greater in acidic solution. The influence of pH also depends
on the composition of the alloy as seen in Figure 1.4. When the zinc is present
with mercury amalgam, the corrosion current is lower than when the metal is
zinc alone. When zinc is present along with platinum, high corrosion rates are
observed as platinum provides effective cathodic sites for hydrogen evolution.
In addition to this, the stability of the passive film in acid, neutral, or alkaline
pH is a contributing factor. Some examples are the stability of magnesium flu-
oride in alkaline medium and the amphoteric nature of aluminum oxide in pH
of 4–8 solutions.
(iii) The difference in temperature can create a corrosion cell in the case of copper
tubes. In general, increase in temperature results in increased corrosion rate.
∘
The corrosion rate of steel in acid solutions doubles for an increase of 10 C