Page 18 - Chemical and process design handbook
P. 18
Speight_Part 1_A 11/7/01 3:04 PM Page 1.4
1.4 REACTION TYPES
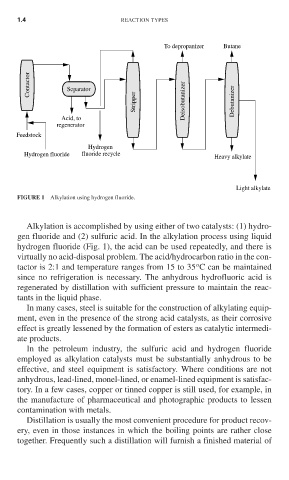
To depropanizer Butane
Contactor Separator
Stripper Deisobutanizer Debutanizer
Acid, to
regenerator
Feedstock
Hydrogen
Hydrogen fluoride fluoride recycle Heavy alkylate
Light alkylate
FIGURE 1 Alkylation using hydrogen fluoride.
Alkylation is accomplished by using either of two catalysts: (1) hydro-
gen fluoride and (2) sulfuric acid. In the alkylation process using liquid
hydrogen fluoride (Fig. 1), the acid can be used repeatedly, and there is
virtually no acid-disposal problem. The acid/hydrocarbon ratio in the con-
o
tactor is 2:1 and temperature ranges from 15 to 35 C can be maintained
since no refrigeration is necessary. The anhydrous hydrofluoric acid is
regenerated by distillation with sufficient pressure to maintain the reac-
tants in the liquid phase.
In many cases, steel is suitable for the construction of alkylating equip-
ment, even in the presence of the strong acid catalysts, as their corrosive
effect is greatly lessened by the formation of esters as catalytic intermedi-
ate products.
In the petroleum industry, the sulfuric acid and hydrogen fluoride
employed as alkylation catalysts must be substantially anhydrous to be
effective, and steel equipment is satisfactory. Where conditions are not
anhydrous, lead-lined, monel-lined, or enamel-lined equipment is satisfac-
tory. In a few cases, copper or tinned copper is still used, for example, in
the manufacture of pharmaceutical and photographic products to lessen
contamination with metals.
Distillation is usually the most convenient procedure for product recov-
ery, even in those instances in which the boiling points are rather close
together. Frequently such a distillation will furnish a finished material of